May 13, 2003— Two factors known to guide patterning of the embryonic nervous system also enable the controlled differentiation of embryonic stem (ES) cells in vitro, report researchers at the RIKEN Center for Developmental Biology in Kobe, Japan. Yoshiki Sasai and his research group, in collaboration with colleagues at Kyoto and Osaka Universities, found that by using BMP4 and Shh they could efficiently induce both mouse and primate ES cells to generate a wide range of neuron types specific to the central nervous system, and, for the first time in primates, peripheral neurons as well.
The study, published in the May 13 issue of the Proceedings of the National Academy of Sciences, builds on previous work by Sasai and colleagues in which they developed a system for inducing ES cells to generate neural cell types. In that system, named SDIA (for Stromal cell Derived Inducing Activity), ES cells are cultured on a bed of feeder cells, called PA6 cells. An as-yet unidentified molecular factor presumed to be present on the surface on the feeder cells causes both mouse and monkey ES cells to adopt neural fates at high to very high efficiencies – in mouse, more than 90% of the ES cells so treated generate neural precursors after four days. The cells generated by this method go on to display the molecular markers characteristic of a central nervous system neural cell types, including dopamine-producing neurons.
|
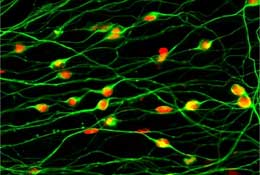
Primate sensory neurons derived from ES cells |
The most recent experiments set out to address the question of whether the neural differentiation of ES cells could be guided with even greater precision and diversity by adding patterning factors – soluble molecules that work to determine the identities of cells in a developmental region – to the SDIA-cultured cells. Using BMP4, Sasai’s group succeeded in causing the SDIA-treated ES cells to differentiate into cell types that normally derive from the neural crest, a developmental region that gives rise to nerve cells in the peripheral nervous system, including sensory, autonomic and enteric (gut) neurons. Although BMP signals have an inhibitory effect on neural development in earlier embryonic stages, the same signals work to promote the neural crest at a later stage in development. The group found that they were able to induce peripheral neural types that are naturally derived from the neural crest, including autonomic and sensory neurons and smooth muscle cells when they treated the SDIA-treated cells with median concentrations of BMP4. A lower concentration prompted the same cells to differentiate into dorsal central nervous system neurons.
This last finding is of interest for its potential in providing a stem cell-based therapy for Hirschsprung’s disease, a congenital disorder afflicting about 1 in 5,000 newborn children in which smooth muscle ganglia fail to develop in the intestine, resulting in a loss of bowel motility and chronic constipation and bowel obstruction. Mild cases are surgically treatable, but the prognosis for the most severe cases is poor. If it is found that human ES cells can also be induced to generate these ganglia in culture, it may one day be possible to use them to remedy the condition by replacing the missing cells.Similar strategies might be applied to the treatment of sensory degenerative diseases, such as chronic neuropathies, if human peripheral sensory neurons can be generated from ES cells in culture.
The neural crest is the dorsalmost (located closest to the back) region of the developing nervous system, and BMP4 is regarded as a ‘dorsalizing’ factor. Other factors have an antagonistic effect to BMP4, and cause the cells they act upon to take on a ventral (or belly-side) character. Peripheral neurons are generally dorsal in nature, while much of the central nervous system derives from more ventral developmental regions. Normal development involves the constant, dynamic interplay between patterning factors that steer neural precursors toward either a ventral or dorsal fate.
|
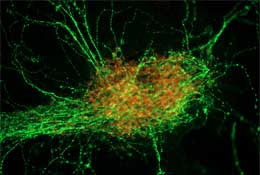
Primate motoneurons derived from ES cells |
Sasai’s group next investigated the effects of Shh (Sonic hedgehog), a factor that suppresses dorsal and promotes ventral development in vivo. As with BMP4, Shh provokes dose-dependent differentiation in SDIA-cultured cells, analogous to its function in the body. A low concentration guides SDIA-derived neural precursors to develop into motor neurons, while higher concentrations brought about differentiation into neural types that in normal development would derive from the floor plate, the ventralmost region of neural development. Tests revealed that these SDIA and Shh-treated neurons displayed both the molecular markers and physiological attributes (such as directional axon guidance) characteristic of the same types of neurons in vivo, demonstrating that the cells produced by this method are structurally and functionally similar to natural floor plate cells.
Taken together, these findings show that SDIA-generated neural precursors respond to patterning factors with a versatility similar to that of their in vivo counterparts. Higher concentrations of the dorsalizing factor BMP4 induce increasingly dorsal neural types, while greater doses of Shh have the same effect in ventralizing neural precursors. This study represents the first efficient generation of such a broad spectrum of neuronal types from ES cells in culture, and the fact that the process has been demonstrated effective not only in mouse, but also in primate cells, marks a significant step toward the ability to induce specific types of neuron from ES cell-derived precursors that is one of the foremost challenges confronting researchers in regenerative medicine.
|