| RIKEN Center
for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
| There and back again: A new mechanism in the regulation of mesenchymal-epithelial transitions | ||||
September 14, 2004 – Epithelium and mesenchyme represent two extremes in the organization of groups of cells. Epithelial cells array themselves into flat sheets or rolled tubes, while mesenchymal cells appear less coordinated in their structure-forming activities and form fewer and looser connections with other cells. These tissue-level differences are reflected at the level of the individual cell as well. Under the microscope, mesenchymal cells are amorphous and lack the distinct apical-basal polarity that characterizes their epithelial counterparts. Both types of cells contribute to the body’s function in distinct ways, with epithelium being the essential structural and physiological component of organs and tissues such as the kidney and the lining of the gut, and mesenchyme forming all migratory cells, including metastatic cancer cells, as well providing support for the epithelium in various contexts. Despite (or perhaps because of) these differences, our anatomy contains countless examples of interaction between epithelial and mesenchymal cells. Indeed, nearly every one of the body’s structures, from internal organs to limbs to teeth, is made up of a mesenchymal and an epithelial component. Developmental biologists are particularly interested in the ability
of each of these cell types to be converted into its counterpart,
epithelial cells become mesenchymal, while in other situations the
reverse transformation occurs. Now, Yoshiko
Takahashi (Team Leader, RIKEN CDB Laboratory for Body Patterning;
Kobe, Japan) and colleagues report a heretofore unknown mechanism
by which mesenchymal-epithelial transitions (METs) are regulated
in the embryogenesis of the chicken. The article, published in the
September 14 issue of Developmental Cell, describes how a pair of
intracellular molecules affects the ability of mesenchymal cells
to convert into epithelial cells during the formation of somites
early in embryogenesis. Somites are transitory structures that appear
in a head-down direction in strictly timed and ordered pairs as
the chicken develops, dividing the embryo into body patterning segments,
and later giving rise to the vertebrae, ribs and the muscles of
the trunk.
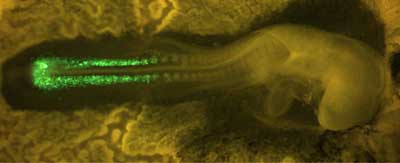
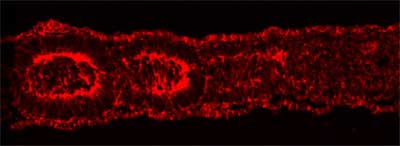
The presomitic mesoderm, from which somites form, consists of a pair of strips made up of mesenchymal cells running down the embryo’s back. After the boundaries of an individual somite have been established, the cells in the border region undergo a transformation, acquiring the polarized structure and properties of epithelial cells. This results in a somite body in which an outer layer of epithelial cells surrounds a mesenchymal core. Takahashi et al. focused on this mesenchymal-epithelial transition to study how mesenchymal cells known for their formlessness are able to make the transition to become highly organized epithelial cells. They used a method that they had previously developed for introducing DNA into somitogenic cells by electroporation to study the role of a group of molecules known as Rho-family small GTPases. This family, which includes Rho, Rac and Cdc42, is known to play an important role in the dynamic organization of the cytoskeleton, the framework of elements that determines many aspects of a cell’s morphology and structure.
The team found that the expression of constitutively active Cdc42 prevented mesenchymal cells from undergoing their normal epithelialization, while interference with Cdc42 function by a specific inhibitor caused an unusually high number of the mesenchymal cells to take on epithelial characteristics, demonstrating the central role of this GTPase in somitogenic MET. These findings were made possible by a novel experimental system developed in the Takahashi lab that takes advantage of the ability to introduce cDNA along with a fluorescent protein directly into a living embryo’s cells by momentarily and reversibly disrupting their outer membranes by the application of a tiny electrical current, a process called electroporation. This technology allowed the team to track cells that had been electroporated with the mutant forms of Cdc42 and observe as they contributed to somite formation. Electroporation of dominant negative and constitutively active forms of Rac1 also indicated a role for that molecule in the mesenchymal-epithelial transition. Rac1 appears to act in a tightly constrained dose-dependent fashion to regulate MET, as both constitutively active and dominant negative forms of the protein disturb somitic epithelialization. Takahashi and colleagues further demonstrated that developing somites in the chicken are able to activate Rac1, suggesting a function for the protein in normal somitogenesis. When they extended their study to investigate possible crosstalk between Rac1 and Paraxis, the only transcription factor known to be essential for somitic MET, they found that Paraxis does indeed require Rac1 in its function as an epithelialization factor, strengthening the argument for its importance in the somitogenic process. The development of a system for introducing DNA directly into somite-forming
cells and the demonstration of the central role played by cytoskeleton-regulatory
molecules in the cell shape changes characteristic of the mesenchymal-epithelial
transition represent significant steps toward a clearer comprehension
of how cellular reorganizations are achieved during development.
And with epithelial-mesenchymal transitions featuring in fundamental
biological processes from organ development to cancer metastasis,
such knowledge may one day contribute to our ability to understand
these processes, and perhaps even to right them, when they go awry.
|
||||
|
||||
[ Contact ] Douglas Sipp : sipp@cdb.riken.jp TEL : +81-78-306-3043 RIKEN CDB, Office for Science Communications and International Affairs |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |