| RIKEN Center for
Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
| Two wrongs make a right: How one mutation compensates for another in guiding cell migration | ||
November 23, 2004 – The developing embryo is a highly trafficked zone, with primordial tissues, individual cells and cellular processes traveling purposefully as they form the body’s structures. Fortunately, these itinerants don’t need to rely entirely on their own sense of direction; the embryonic landscape is clearly marked with molecular signposts that migrating cells where and where not to go.
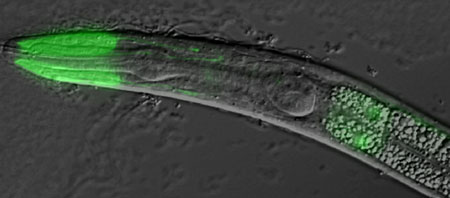
The Nishiwaki had its first clue to the guidance function of the protein from a genetic screen they conducted to identify mutations that suppressed migration defects in mig-17 null mutants. The screen uncovered a pair of mutations that allowed normal DTC migration in the absence of MIG-17; these were subsequently identified as mutants of the gene fbl-1, a C. elegans homolog related to the mammalian fibulins, a family of genes that encode proteins associated with the basement membrane, extracellular matrix and blood plasma. Closer investigation of the fbl-1 mutants revealed that only one of two isoforms of the protein, FBL-1C, rescued the MIG-17 deficiency phenotype. Protein isoforms are naturally occurring variant configurations of gene products; in the case of FBL-1, the isoforms FBL-1C and FBL-1D differ only in their C-terminal domains. Nishiwaki et al found that when they induced a gain of FBL-1 function by inserting multiple copies of mutant genes into mig-17 mutants, they partially restored DTC migration, a finding that suggested the mutant protein functions to enable proper migration, in contrast to the wild type, which interferes with the process in mig-17 deficient worms. Analysis of distribution patterns of both FBL-1 isoforms confirmed that FBL-1C localizes in the gonadal basement membrane, while FBL-1D does not, shoring up the case for a specificity of action to gonadal guidance. Interestingly, the basement membrane localization of wild type FBL-1C was dramatically reduced in mig-17 mutants, while the isoform of a mutant version of the protein was unresponsive to MIG-17 activity. These findings permit a number of interpretations of the role of fbl-1 in mig-17 control of cell migration. The simplest model is one in which FBL-1 is a direct target of MIG-17; the results of Western blots for FBL-1 in wild-type or mutant mig-17 animals, however, tend to argue against this. It seems more likely that MIG-17 acts on an as-yet unidentified intermediary substrate, which affects the affinity of the gonadal basement membrane for FBL-1C. Isoforms from the fbl-1 mutant are immune to the effects of MIG-17, and appear to mimic the effects of MIG-17 activity on the gonadal basement membrane in its absence. The exact mechanisms by which FBL-1 interacts with MIG-17 and other ADAM-family proteases remain to be worked out, but the Nishiwaki study indicate a new role for fibulins in cell guidance, as well as illustrating that, even at the genetic level, sometimes multiple errors can lead to a correct result.
|
||
|
||
[ Contact ] Douglas Sipp : sipp@cdb.riken.jp TEL : +81-78-306-3043 RIKEN CDB, Office for Science Communications and International Affairs |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |