| RIKEN Center for
Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
| Crest commencement: Pax3 and Zic1 co-activate neural crest differentiation | ||
April 19, 2005 – Early in vertebrate development, the foundations of the nervous system are laid down in specific regions of the embryonic body. A sheet of epithelial tissue rolls into a cylinder, forming the neural tube, the structure that will give rise to the central nervous system. A migratory population of cells called the neural crest develops slightly later, before spreading throughout the body to create the peripheral and autonomic nervous systems, as well as a range of other tissues including facial cartilage and bone, the pigmented cells called melanocytes, and the adrenal medulla. Despite the importance of the neural crest, however, the molecular signals that function upstream in the multistep process of the specification and demarcation of its developmental field have remained a mystery.
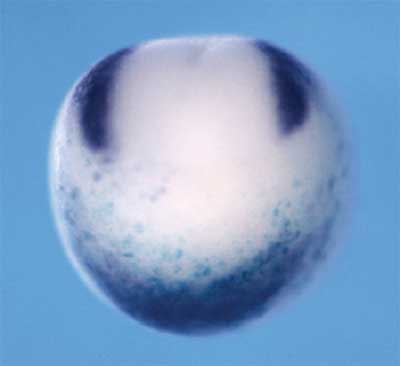
A host of regulatory molecules, including members of the BMP and Wnt signaling families, have been implicated in this determination process, and a pair of transcriptional factors, Foxd3 and Slug, has been identified as definitive markers of the presumptive neural crest, but the factors that define its exact boundaries have stayed out of reach. Now, in a study published in the online edition of the journal Development, Yoshiki Sasai (Group Director, Laboratory for Organogenesis and Neurogenesis) and colleagues in the RIKEN CDB (Kobe, Japan) report the identification of a pair of overlapping regulatory signals that seem to initiate the neural crest developmental program in the African clawed frog, Xenopus laevis. Earlier studies in the same laboratory had suggested a role for Zic-family factors, and they focused on Zic1, which is expressed in the dorsal ectodermal region of the gastrulating embryo, the site of prospective neural development. A second molecule, Pax3, shows a similar but distinct pattern of expression in about the same region and embryonic stages, leading the Sasai group to narrow their search to these candidates. Preliminary tests showed that an increase in BMP signaling, a potent neural inhibitor, suppressed the expression of both, while the suppression of BMP caused an expansion of their range toward the ventral side of the embryo. Conversely, the soluble factor Wnt caused Pax3 and the presumptive neural crest marker, Foxd3, to be expanded beyond their normal anterior limits. They next looked at the effects of gain of Pax3 and Zic1 function in the developing frog, and found that both were able to trigger neural crest differentiation, as evidenced by the expression of Foxd3 and Slug prior to the late gastrula stage, when those markers normally first appear, as well as in the typically non-neural ventral region. When misexpressed singly, both Pax3 and Zic1 showed the ability to trigger an ectopic expansion of Foxd3 and Slug in the dorsal region, but that effect did not extend to the ventral side. On direct injection of both Pax3 and Zic1 into the ventral side of animal blastomeres from very early embryos they found that the factors in combination could indeed induce neural crest markers even in the ventral side, indicating the potency and directness of their effect. Sasai et al followed up by studying how a loss of these molecules’ function might affect the neural crest in otherwise normal embryos by injecting morpholino (MO) antisense oligonucleotides (a method of inhibiting the function of specific genes by interfering with the translation of the proteins they encode). The injection of either Pax3 or Zic1 MOs was sufficient to suppress the expression of the marker Foxd3, while the loss of function of either of the two factors had no discernible effects on the expression of the other, suggesting that both must be active to achieve normal determination of the neural crest. Animal cap assays, which provide an in vitro model of many aspects of early Xenopus development, helped to clarify the details of the molecular interactions at work. Finding that Pax3 alone failed to induce Foxd3, as it had in vivo, they began to search for the missing signals needed to achieve that effect. When they co-injected Wnt3a (a known factor in neural crest differentiation), they found not only that Pax3 now strongly induced Foxd3, but also that Zic1 began to be expressed. Injection of Zic1 alone into untreated animal caps was able to induce Foxd3, but only weakly, an effect that was strongly complemented by co-injection with Wnt3a. Interestingly, the inductive action of these factors acting alone could be blocked by increasing the activity of the neural inhibitor, BMP4, but the combination of Pax3, Zic1 and Wnt3a proved able to induce Foxd3 robustly even in the face of an antagonistic BMP signal. By interfering with gene function in dissociated cells, the group tested whether this co-activity between Pax3 and Zic1 in Wnt treated cells relied on external signals. Morpholino blockade of Zic1 in Pax3-injected and Wnt-treated single cells resulted in the loss of Foxd3 induction, while cells exposed to all three signals continued to express Foxd3, indicating that the Pax3, Zic1 and Wnt3a effect is cell-autonomous. The critical role of the endogenous Wnt cascade was shown by the loss of Foxd3 induction when Wnt signaling was disrupted by morpholino knockdown of β–catenin, a Wnt downstream factor. This comprehensive and compelling set of evidence points strongly to a modus of neural crest differentiation involving the close cooperation between Pax3 and Zic1 in the presence of Wnt signaling in the pre-neural embryo. That this trio of signals operates even in the presence of inhibitory BMP signal suggests that the combination is a powerful determinant of the prospective neural crest, and the question of exactly how the Pax3-Zic1 partnership overrides BMP on the molecular level represents an intriguing question for further study.
|
||
|
||
[ Contact ] Douglas Sipp : sipp@cdb.riken.jp TEL : +81-78-306-3043 RIKEN CDB, Office for Science Communications and International Affairs |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |