| RIKEN Center for
Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
| Poles apart: Asymmetry of cortical and nuclear localization of WRM-1 | ||||
August 1, 2005 – The body’s great wealth of cellular diversity is generated by round after round of cell divisions in which a parent cell gives rise to a pair of progeny of differing types, in a process known as asymmetric cell division. This can be brought about in a number of ways, one of the most common of which is the segregation of components integral to the determination of cell fate to different regions of the dividing cell. On mitotic division, these localized cell fate determinants are apportioned only to one of the two daughters, influencing its essential character and setting it apart from its sibling. In a sense, although such cells share the same genome, in their appearance and behavior they are more akin to fraternal than identical twins. In many species, the Wnt signaling pathway – a molecular routine used to regulate the transcription of certain genes – is known to play a role in instructing cells to divide unequally. This widespread role, however, manifests in subtly different ways in different taxa. The roundworm, C. elegans, features a version of the Wnt cascade that includes WRM-1, a homolog of the transcription factor, β-catenin. In species from fly to man, this molecule is a dual agent, serving important functions in both cell adhesion, in which it forms a complex with transmembrane cadherins, and Wnt signaling, where it its accumulation and degradation are determined by upstream events governed by the binding (or failure to bind) of Wnt to its receptor at the cell membrane. The roundworm homolog WRM-1, however, does not appear to follow this general pattern – it has no known role in cell adhesion, it may not be phosphorylated by GSK3 as in other species, and its intracellular localization or its function in the regulation of cell polarity haveremained obscure. Now, in an article published in the August 1 issue of Genes and Development, Hisako Takeshita and Hitoshi Sawa (Team Leader, Laboratory for Cell Fate Decision) describe an intriguing pattern of localization for WRM-1 and a second molecule, the MAP kinase homolog LIT-1, before and after asymmetric mitosis in C. elegans cells that helps to clarify the picture of roundworm cell polarization.
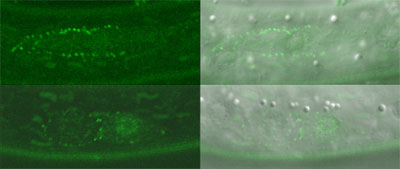
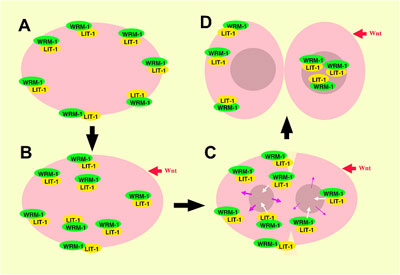
C. elegans cell divisions follow highly stereotyped and well-characterized patterns, making it relatively simple to detect aberrations from the norm. Using several strains of worms in which a region required for the function of the wrm-1 gene had been deleted or conditionally interfered with, Takeshita and Sawa looked at cell division in these animals and found disruptions in the typical patterns of cell progeny. Similar results were obtained in studies of cell divisions in multiple cell lineages in mutants for the gene lit-1, or is activator mom-4, indicating that the majority of post-embryonic cell divisions in C. elegans are regulated by a set of similar mechanisms. They next fused a gene encoding green fluorescent protein (GFP) to wrm-1 and lit-1 to allow them to visually track the sites to which their protein products localized. In many of the cell pairs, they found that the nucleus of the posterior daughter showed a higher WRM-1 or LIT-1 GFP readout. However, during cell division, including telophase, the stage at which mitotic division nears completion and the two nuclei are cordoned off into opposing halves of the dividing cell, both WRM-1 and LIT-1 could also be detected at the anterior cortex. Interestingly, WRM-1 was also seen in both nuclei at this stage, suggesting that it is exported from the anterior nucleus during the latest phase of mitosis. Curious to know more about how this shift in distribution occurred, the Sawa team used a technique known as fluorescence recovery after photobleaching (FRAP), in which all or part of a GFP-tagged cell is flashed with a bright light capable of eliminating its fluorescence, and then monitored to determine when and where its green glow is restored. After photobleaching the entire cell early in mitosis to show that GFP activity is not recovered when universally bleached away, they next applied light bursts to the anterior half of the cells, or to the posterior half of cells undergoing division, and found that in either case the recovery of both WRM-1 and LIT-1 fluorescent reporter activity was limited to the nucleus of the resulting cellular progeny – a sign that both the anteriorly or posteriorly localized moieties of these factors ultimately make their way to the posterior nucleus. Subsequent tests lent further weight to the idea that, in the case of LIT-1 at least, the redistribution was achieved by a higher rate of export from the anterior nucleus.
Perhaps their most interesting finding came when they looked at WRM-1 and LIT-1 dynamics in a mutant for the wnt homolog, egl-20. In these mutants, prior to division the localization of WRM-1 and LIT-1 in the cortex became randomized, but by telophase these factors always localized to the nucleus on the opposite side of the dividing cell, regardless of the cortical starting point. Cross testing showed that while WRM-1 was required for localization of LIT-1 to the cortex and nucleus on opposite sides of the mitotic cell, the MOM-4 LIT-1 pathwayseems to regulate only the nuclear localization of WRM-1. |
||||
|
||||
[ Contact ] Douglas Sipp : sipp@cdb.riken.jp TEL : +81-78-306-3043 RIKEN CDB, Office for Science Communications and International Affairs |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |