| RIKEN Center for Developmental Biology
(CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
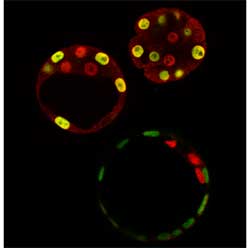
A significant portion of that puzzle has now been fit into place by researchers from the Laboratory for Pluripotent Cell Studies (Hitoshi Niwa; Team Leader), who describe the interaction between a pair of factors, Oct3/4 and Cdx2, that sets up the trophectoderm/ICM divide. Working with colleagues from Kobe University, the Japan Science and Technology Agency and Mount Sinai Hospital (Canada), Niwa puts forth a new model for the genetic basis of the earliest instance of cellular differentiation in the December issue of the journal Cell. In previous work with embryonic stem (ES) cells, which share many of the properties of cells from the blastocyst interior, Niwa discovered that he could induce the ES cells to differentiate into trophectoderm (something they do not normally do) by repressing the function of the transcription factor, Oct3/4. Other work has further recently identified a second molecule, Cdx2, as intimately involved in trophectoderm formation in the mouse blastocyst. With that finding as a cue, the Niwa team tested the effects of overexpressing Cdx2 in ES cells in vitro and found that this also could induce trophoblast stem (TS) cells under certain culture conditions. On injection back into a blastocyst, Cdx2-induced TS cells that had been labeled with a fluorescent transgene to allow them to be visualized were seen to give rise to placental cell lineages in the chimeric embryos, as evidenced by the green glow from the placenta when exposed to an excitation wavelength of light. Prompted by their discovery to investigate the possibility of a relationship between Oct3/4 and Cdx2 (specifically, that they might act in a mutually inhibitory fashion), the team transfected ES cells with both molecules and observed the effects on genes normally activated by endogenous Oct3/4. They found that Cdx2 represses Oct3/4’s transcriptional activating effects and, interestingly, that this repression depended on Oct3/4; in the absence of Oct3/4, no Cdx2-mediated inhibitory effect was seen on its downstream target genes. Intriguingly, Oct3/4 seems to exert a suppressive influence on Cdx2 as well. Cdx2 is thought to positively regulate its own expression in ES cells, but when these same cells are cultured with an Oct3/4 expression vector, this autoregulation is significantly damped. This set of findings hinted that Cdx2 and Oct3/4 are locked in a mutually inhibitory relationship. Curious about the mechanics of this interaction, they first labeled the two molecules to study their localization within cells and found that when both were expressed together, they relocated to inactive regions of the nucleus. Next, they performed immunoprecipitation analysis (a means of determining whether two molecules bind to each other) and found that Oct3/4 and Cdx2 do indeed co-precipitate, indicating a direct interaction on the molecular level. The story took on a new twist when Niwa et al looked at ES cells lacking both Cdx2 and Oct3/4 function. Although Cdx2 was thought to be an induce of trophectoderm, they discovered that, when Oct3/4 function is inhibited, even ES cells with homozygous deletions of Cdx2 can give rise to trophectoderm. The unexpected dispensability of Cdx2 in trophectodermal differentiation led them to inquire just what does Cdx2 actually do. Creating a line of cells in which Cdx2 expression could be controlled conditionally, they compared TS cells derived solely as a result of Oct3/4 donwregulation with those actively induced by Cdx2 expression, and found that the TS cells lacking Cdx2 were deficient in their ability to self-renew, which is one of the hallmark properties of all stem cells. As it turns out, a second factor, Eomesodermin (Eomeso), is capable of inducing trophectodermal differentiation even in the absence of Cdx2; Eomeso, however, could not entirely compensate for Cdx2 function, and had no repressive effect on Oct3/4.
|
||||
|
||||
[ Contact ] Douglas Sipp : sipp@cdb.riken.jp TEL : +81-78-306-3043 RIKEN CDB, Office for Science Communications and International Affairs |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |