| RIKEN Center for Developmental Biology
(CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
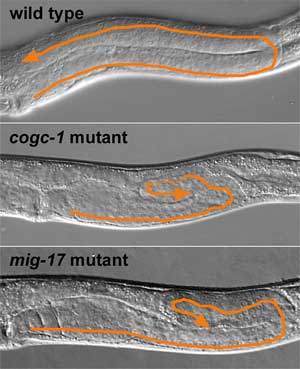
The Nishiwaki lab had previously shown that the ADAM protease MIG-17 is secreted from the body wall musculature in larvae and localizeat the gonadal basement membrane, where it promotes the migration of distal tip cells. A pair of other genes, mig-29 and mig-30, identified in the same mutation screen that yielded mig-17 were found to code for homologs of components of the conserved oligomeric Golgi (COG) complex, which functions in many aspects of vesicle trafficking within the cell and has been identified in species from yeast to human. The genes were renamed cogc-3 and cogc-1 to reflect this homology, and Kubota et al set out to analyze their role in DTC migration. In other species, the COG complex comprises eight protein constituents structured as a pair of lobes, which was shown to be true in C. elegans as well. The Nishiwaki team then demonstrated the functional importance of each of the COG components by showing that interference by RNAi with the function of any of the eight COGs produced defective DTC migration or malformed gonad phenotypes, an effect that was found to be stronger for lobe A COGs (which includes the proteins encoded by both cogc-1 and -3) than for those in lobe B. This led Kubota to the possibility that the COG complex might interact with other factors known to be involved in DTC migration, specifically to MIG-23, a Golgi membrane protein the lab’s previous work had identified as a regulator of MIG-17 via the addition of sugar residues, a process known as glycosylation. COGC-3 was observed to co-localize with MIG-23 in muscle cells of the body wall, and when the team looked at cogc-3 and cogc-1 mutants, they found that MIG-23 was destabilized and decreased in quantity. Following the evidence trail, they looked next to MIG-17 glycosylation in these mutants and again found that its glycosylation was incomplete resulting in its failure to accumulate at the gonadal basement membrane. The body of findings from these studies prompted the Nishiwaki lab to develop a picture of the molecular mechanisms underlying DTC guidance in which the COG complex functions to stabilize MIG-23, thereby ensuring the glycosylation of MIG-17 in muscle cells, which then secrete it to remodel the adjacent basement membrane and so steer the distal tip cells on their parabolic pathways. Their work provides the first evidence of the function of the COG complex in an organ-forming process, which is of some biomedical interest as well, as humans carrying a mutation for another COG component, COG-7, develop a glycosylation disorder characterized by multiple developmental defects. Here, as in many aspects of development, what is true in the worm may one day prove to be equally important in man.
|
||||
|
||||
[ Contact ] Douglas Sipp : sipp@cdb.riken.jp TEL : +81-78-306-3043 RIKEN CDB, Office for Science Communications and International Affairs |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |