| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
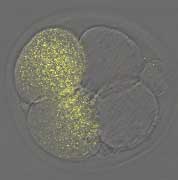
Now, in an article published in the EMBO Journal, Shisako Shoji and colleagues from the Laboratory of Mammalian Molecular Embryology (Tony Perry; Team Leader) report that the classical experiment of Masui and Markert does not hold entirely true in mice, despite the fact that over the years its findings in amphibians had become firmly established frogma. Prompted by this incongruity, the team developed an alternative approach to the problem, one that utilized RNA interference (RNAi) to remove selected oocyte transcripts and proteins, allowing them to attack the problem systematically on a molecule-by-molecule basis. Surprisingly, depletion of the previous CSF candidates Emi1 or Mos had little or no effect.But when they knocked down the oocyte component, Emi2, they found that oocytes clearly progressed through the cell cycle without arresting at meiotic metaphase II (mII), the phase in which the mature oocyte normally remains prior to its activation at fertilization. When the group looked more closely at older, non-manipulated oocytes, they found that Mos almost entirely disappeared autonomously, even though the oocytes remained at mII; RNAi was not necessary. The group found that Emi2 is necessary not only to establish mII arrest,but also to sustain it.Emi2 removal causes cell cycle progression with unexpected embryonic degeneration, suggesting that it links the meiotic cell cycle with cytokinesis (cell division, which in this case occurs asymmetrically to produce a single cell embryo plus a second polar body). Following this thread, Shoji et al. next showed that Emi2 works through Cdc20, an adaptor of the anaphase promoting complex (APC). The APC is a multiprotein complex that works by blocking the activity of the cyclin-containing kinase (MPF), whose presence is ultimately responsible for maintaining the state of mII arrest prior to fertilization. It appears that Emi2 arrests the oocyte at mII by inhibiting the degradation of MPF, and that it achieves this by binding to Cdc20. The team then looked at both parthenogenetically activated oocytes and those activated by fertilization and found that MPF was eliminated in a Cdc20-dependent manner. “This is probably the first formal demonstration that the signaling responsible for MPF ablation has molecular components common to both parthenogenesis and fertilization,” Perry notes, “which is reassuring given the application of parthenogenetic activation in nuclear transfer and other current research.”
|
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |