| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Now, in a study published in the March issue of the journal Developmental Cell, Hitoshi Sawa (Team Leader; Laboratory for Cell Fate Decision) and colleagues from Kobe University and at the University of North Carolina at Chapel Hill (USA) have revealed that Wnt signals can determine the polarity of both embryonic and postembryonic cells in the nematode C. elegans. These findings stand in contrast to previous studies in which misexpression of Wnt proteins suggested that Wnt signals may only need to be present, while other signals actually effect the polarization.
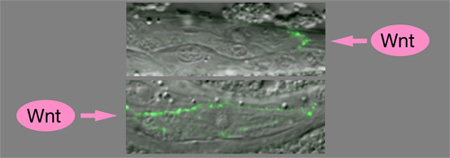
The team next devised a series of tests in which a pair of altered signaling cells (one lacking MOM-2, the other lacking MES-1) were placed on either side of a responding cell allowing them to study the effects on endoderm development, which normally occurs in a responder exposed to both signals. In their initial experiments, endodermal development proceeded as usual, so they next tested placing the Wnt factor MOM-2 and the (non-Wnt) MES-1 cells in different positions relative to each other, and found that the orientation of the mitotic spindle (a cytoskeletal structure that pulls the chromosomes of a dividing cell apart) was consistently oriented in line with the source of the Wnt signal. Wnt signaling had also been known to play some role in the establishment of polarity in postembryonic cells, but here again, they faced the question of whether a Wnt factor (this time, LIN-44) serves only in a permissive capacity in the regulation of polarity, or whether it acts as a true positional determinant. By ectopically expressing LIN-44 and examining the effects on asymmetric cell division of T cells found in the worm’s tail, Sawa et al hoped to find evidence of Wnt’s true function. In normal development, LIN-44 is usually expressed in cells posterior to the T cells; the polarity of these cells is often reversed when this factor is absent. By misexpressing LIN-44 in cells anterior to T cells the authors observed strong enhancement of the polarity reversal phenotype in lin-44 mutants. Once more, the position of the Wnt signal source was shown to play a critical role in determining cellular sidedness, this time at a postembryonic phase of development.Sawa et al further showed that the Wnt signal determines the polarized localization of the Wnt receptor LIN-17/Frizzled in the T cell, indicating that its polarizing effect in these cells is direct. |
|||||
|
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |