| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
The question of how microtubules might determine the site of the nascent contractile ring has remained an open one for some years. One theory is that astral and/or central microtubules help to focus or hold the ring at the center of the cell, such that it forms perpendicular to the mitotic spindle pulling the chromosomes laterally to the poles. A second conjecture is the cleavage furrow forms due to a relaxation of tension at the cell’s cortex at its poles, while a third ascribes a more actively inhibitory role to microtubules on cortical relaxation, such that the cleavage furrow is prevented from forming anywhere other than at the central plane of division.
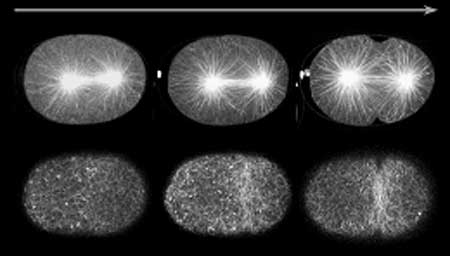
Now, in an article published in the April issue of Developmental Cell, Fumio Motegi and colleagues in the CDB Laboratory for Developmental Genomics (Asako Sugimoto; Team Leader) and New York University (USA) report findings that point to a new model for astral microtubule activity during cytokinesis. Working with the nematode C. elegans, Motegi finds that astral microtubules, which localize on opposing sides of the mitotic nucleus and help draw the chromosomes away from each other, in fact exhibit two separately mediated modes of action; one that localizes the contractile ring to the cell’s equator, and a second that suppresses furrowing at all other regions of the cortex. “This is something of a reconciliation between a pair of rival theories, in the sense that Fumio was able to show that cortical relaxation and contractile ring induction, both of which are regulated by microtubules, could play complementary roles in a single dividing cell,” says Sugimoto. Early in the study, the team concentrated on the activity of astral microtubules at the cell cortex and, using GFP labeling to visualize the cells, they observed that these microtubules tended to behave differently at different stages of mitosis. In early anaphase, when the chromosomes are just beginning to separate, microtubules appeared had dots or comet-like in appearance, while in late anaphase, when the cleavage furrow begins to divide the cell into halves, the microtubules were filamentous and persisted longer. They also found that microtubules were most densely concentrated in the part of the cell where the contractile ring arises, and that, indeed, the early anaphase concentration of microtubules at the spindle equator appeared to promote the accumulation of RHO-1 (necessary for contractile ring formation). A focused gene screen delivered them a trio of candidates for further study: tbg-1, which codes for γ-tubulin, gip-1, whose protein product interacts with γ-tubulin, and air-1, encoding aurora-A kinase. Interfering with the functions of these genes by RNAi, revealed very different patterns of phenotype. In the absence of γ-tubulin, the cleavage furrow frequently failed to form, whereas on air-1 knockdown, the contractile ring assembled correctly at the central axis, but the cells later tended to exhibit furrow-like ingressions in multiple incorrect sites as well. |
|||||
|
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |