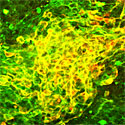
| Clinically safe culture of human ES cells |
 |
 |

June 10, 2006 – Much has been said about the great potential of embryonic stem (ES) cell-derived cells as a material resource for regenerative medical therapies. If such cells can be grown in culture in a safe and controlled fashion, and induced to differentiate into populations of specified human cell types, it would represent a significant step toward realizing the dream of generating replacement cells for bodies damaged by injury, disease or age. But, as with any clinically-oriented work, precautionary principles must be observed prior to the translation of findings from basic research into applications in human patients. For human ES cells, one of the primary concerns has been that most extant culture methods entail the use of non-human biological material to support growth and induce differentiation.
Now, in an article published in the June 9th Early Edition of the Proceedings of the National Academy of Sciences USA, Morio Ueno of the Laboratory for Organogenesis and Neurogenesis (Yoshiki Sasai; Group Director) and colleagues report a new clinically-safe method for cultivating and steering the differentiation of human ES cells in vitro. This new method, which the authors of the study named AMED (for amniotic membrane matrix-based ES cell differentiation), uses only biological materials of human origin and demonstrates efficiencies similar to those of previously published methods in inducing ES cell differentiation into a range of neuronal cell types. The method was developed in collaboration with other CDB researchers, as well as labs in Kyoto Prefectural University of Medicine and Kyoto University, and was funded in part by the MEXT “Leading Project in Regenerative Medicine.”
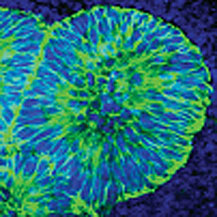 |
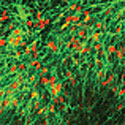
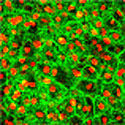
| Neural precursors derived from AMED-treated ES cells |
Several methods for driving ES cells to differentiate into specialized neurons characteristic of the central nervous system have been published in the past, including a technique first reported by the Sasai lab in 2002 that relies on stromal cell-derived inducing activity (SDIA) exerted by mouse feeder cells, resulting in highly efficient differentiation into midbrain dopaminergic neurons. The clinical potential of these SDIA-induced neurons was evident from experimental trials in which these cells were transplanted into a non-human primate model of Parkinson’s disease (a condition affecting dopaminergic neurons in humans), resulting in dramatic recovery of motor function and neurotransmitter uptake. But in spite of this great promise, cells derived using such methods cannot be transplanted into humans due to the risk of contamination or infection via contact with xenogeneic feeder cells or uncharacterized culture media.
In the present report, Ueno et al found that both mouse and human ES cells can be cultured on a substrate derived from the matrix layers of the human amniotic membrane (hAM; which encases the baby within the mother’s uterus, and which ruptures and is sloughed at birth). This naturally occurring material is already widely used in surgical practice, including various grafting procedures, making it much more acceptable for use in the culture of cells that may one day be transplanted into human patients. The amniotic membrane used in this study were obtained following informed consent from patients undergoing cesarean sections at local hospitals; human ES cells were provided by Norio Nakatsuji’s lab in Kyoto University, which established the line using surplus blastocysts from in vitro fertilization clinics, in accordance with Japanese national regulations.
To prepare the amniotic membrane for use, the group carefully removed the matrix from its overlying epithelium, then transferred it to cell culture plates. Human ES cells grown for two weeks using this AMED method differentiated into Nestin+ neural precursors at an efficiency of approximately 90%, comparable to the purities achieved by the mouse feeder cell-based SDIA. After four weeks, a full 40% of these had differentiated into mature neurons, about a third of which exhibited a dopaminergic phenotype. And importantly, just as had been shown for SDIA, AMED-treated ES cells could be induced to differentiate into a spectrum of central and peripheral nervous system neuronal types, including motor and sensory neurons, and aggregates of retinal pigmented epithelium and the lens cells of the eye, by the addition of growth factors at developmentally appropriate time points.
The Ueno publication stands as a new milestone in the rapid advance of Japanese ES cell research. Building on a method developed for culturing corneal cells on human amniotic membrane by Kyoto Prefectural University of Medicine researchers in 2000, this report represents the culmination of just one and a half years of intensive experimentation, but one whose implications extend well into the future of regenerative medicine. The source of the amniotic membrane matrix’s inductive activity, however, remains obscure. “In some ways, it’s almost satisfying that the molecular mechanism at work here is still a mystery,” remarks Sasai, “but it’s a mystery we’ll be working hard to solve.”
|