| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Now, Yukinobu Arata and colleagues in the Laboratory for Cell Fate Decision (Hitoshi Sawa; Team Leader) report that cell specialization arises as the net regulatory effect of Wnt signaling and positional identity cues on a novel factor, PSA-3. “This is a neat demonstration of how cooperation between a general mechanism for asymmetric cell division and position-specific factors can lead to discrete outcomes during development,” says Arata.
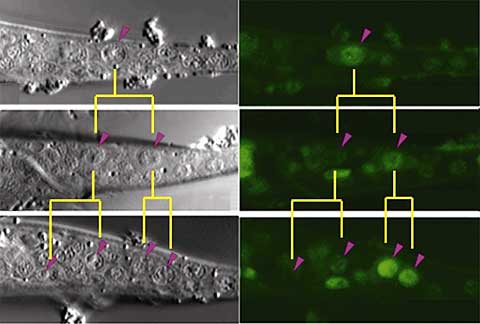
Analysis of its sequence revealed that psa-3 shared similarities with genes in the Meis family of transcription factors, and even more intriguingly, contained a domain that appeared to be a POP-1 binding sequence. The team created a GFP psa-3 construct to allow them to follow its activity in T cells, and found that psa-3 was gradually upregulated in the posterior descendants, following the initial T cell division. In worms engineered with a nucleotide substitution in the putative POP-1 binding sequence, this effect was lost, indicating to the team that psa-3 expression in posterior T cell daughters is regulated by its interaction with the C. elegans Wnt signaling pathway. It remained to be seen, however, how psa-3’s effect could be kept specific to posterior descendants of T cells, when POP-1 was known to operate in the asymmetric divisions of all seam cells, of which T cells are only a subset. Roundworms with mutations in the Hox equivalent nob-1 and the Pbx homolog ceh-20 showed decreases in psa-3 in the posterior lineage. Searching for the regulatory elements responsible, they found that the fourth psa-3 intron contained a binding sequence for NOB-1, and that this interaction appeared to be facilitated by CEH-20, indicating that the tail Hox factor, NOB-1 allows psa-3 to function selectively in posterior descendants of T cells. And interestingly, the Sawa team found that psa-3 itself functioned in localizing CEH-20 to the nucleus in posterior T cells, suggesting a regulatory circuit in which psa-3 and ceh-20 cooperate to maintain each other’s functions. These findings were borne out in vivo, where the results of heat shock rescue experiments indicated that PSA-3, NOB-1 and CEH-20 work as co-factors in regulate cell fate in the posterior lineage after asymmetric T cell division. “It appears that similar mechanisms for asymmetric division are used repeatedly during the development, not only of C. elegans, but many other organisms as well,” notes Sawa. “It will be interesting to find out whether Hox proteins cooperate with a common mechanism for asymmetry to specify diverse cell fates in other species as well.” This study, published in the July issue of Developmental Cell, was made in collaboration with scientists from the Keio and Osaka University (Japan) and Kansas State University (USA), and with funding support from the Japan Science and Technology CREST and PRESTO programs. |
|||||||||
|
|||||||||
|
|||||||||
 |
|||||||||
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |