| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
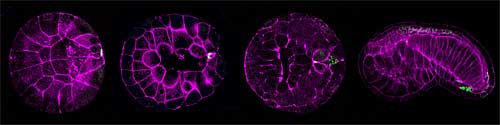
Ascidian postplasm also contains a high concentration of RNAs and the protein product of the CiVH gene (a homolog of the Drosophila germline specific gene vasa, which is essential for germ cell development and highly conserved in vertebrates and invertebrates). These CiVH-positive cells are subsequently incorporated into juvenile Ciona gonads and form the animal’s germ cells. Given these similarities with primordial germ cells in other species, many researchers now believe that postplasm serves as the ascidian germ plasm, in which the progeny of a highly specific subset of blastomeres, termed B7.6 cells, develop into PGCs. A number of postplasmic components, including the centrosome-attracting body itself, are also known to have a role in promoting asymmetric cell division and the regulation of somatic cell differentiation during early embryogenesis. One of these factors, posterior end mark (PEM) RNA, appears to control the positioning of cleavage planes, thereby promoting asymmetric division.Intriguingly, the detection of these postplasmic factors in presumptive B7.6 cells opened up the possibility that, in addition to their known function in somatic cell differentiation, they might also play roles in germ cell formation as well. Previous studies were unclear, however, as to the stage at which B7.6 cells directly merge into the gonads, and whether the postplasmic components involved in somatic cell development do in fact contribute to germ cell formation. Shirae-Kurabayashi et al. first established that Ciona B7.6 cells undergo an asymmetric cell division to produce two daughter cells, B8.11 (the CAB-containing anterior cells) and B8.12 (CAB-negative posterior cells).As most of the postplasmic components are associated with the CAB, these too are partitioned solely into the B8.11 daughters, subsequently losing CiVH protein expression and associating with the animal’s gut wall. The B8.11 cells, however, did retain the remnants of the CAB and other postplasmic components, which were not seen in B8.12 cells, suggesting that B8.11 cells do not function in the specification of germ cells. The team did find, however that maternal CiVH RNA and proteins entered the cytoplasm from the CAB immediately prior to B7.6 division, and were then inherited by the B8.12 progeny. Subsequent upregulation of CiVH protein production in B8.12 cells resulted in the formation of perinuclear CiVH granules, which they tentatively identified as the nuage characteristic of germ cells. Using DiI labeling and CiVH immunostaining, the Nakamura team found that the descendants of the B8.12 cells are incorporated into Ciona primitive gonads and go on to form the germ cells. This suggests that the Ciona B8.12 cells are indeed early germline progenitors, and that formation of a nuage-like structure occurs immediately after the B7.6 cell division, much earlier than was previously thought. germline,” says Shirae-Kurabayashi, “ but we also know that the germline will regenerate itself if it is ablated from a larval ascidian, which suggests that there are two separate mechanisms – embryonic and postembryonic – for constructing the germ lineage in these animals, so of course we’re very curious to find out how each of those works.” |
|||||||||
|
|||||||||
 |
|||||||||
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |