| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
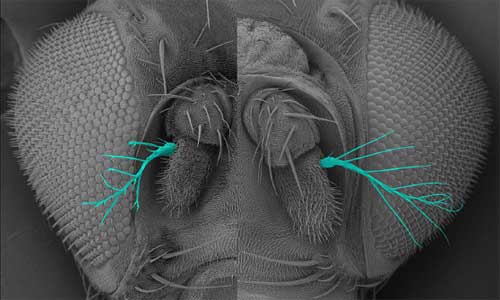
Oshima first noted IKKε in a screen of genes involved in epithelial morphogenesis in the fly trachea, finding that the overexpression of this protein led to epithelial disruption, the loss of apical-basal cell polarity, and the abnormal accumulation of F actin in the apical region of cells. Looking at its expression pattern in wild type cells, the group found that IKKε tended to accumulate in thicker, cytoplasmic regions of cells, rather than at the thinner peripheries, where F actin localizes. Sites where the expression of these two proteins intersected, however, frequently developed the “ruffling membrane” structures characteristic of cell motility. Examining the effects of downregulation of IKKε in vitro, they found that while cells survived even when the gene’s activity was interfered with, the loss or lessening of IKKε function resulted in dramatic changes in cell morphology, with such cells exhibiting a ruffled, serrate or spiky, stellate morphology at much higher frequency than the smooth phenotype typical of control cells. Overexpression had the opposite effect, causing more of the cells to develop smooth edges and, interestingly, to die by spontaneously entering the process of programmed cell death, apoptosis. Analysis of the rates of F actin turnover in IKKε up- and downregulated cells indicated that retrograde flow of F actin from the peripheral edges of the ruffling membrane back toward the cytoplasmic interior was reduced in IKKε-deficient cells. Cell motility and migration is fundamental to numerous morphogenetic processes during Drosophila development, so Oshima and colleagues next looked at the role of IKKε in living embryos, focusing on the formation of the trachea, a branching network of epithelial lumina. In the loss-of-function mutant, the trachea developed normally at first, but in later stage embryos the terminal tracheal branches went haywire, exhibiting defects including misorientation, splitting and duplication at a frequency of about 30%. The authors surmised that these phenotypes were the result of ectopic accumulation of F actin, leading to abnormalities in the morphology and movement of the terminal branches. Similar defects were observed in the development of other extended structures in which F actin assembly is involved, including sensory bristles and the terminal segment of the antennae, known as aristae. Returning to the possible link with apoptotic cell death factors suggested by the overexpression phenotype, the Hayashi group looked for a potential interaction between IKKε and an apoptosis inhibitor, DIAP1, which had previously been shown to negatively regulate IKKε expression. Increased levels of DIAP1 intensified the IKKε loss-of-function dysmorphology phenotype, while overriding the effects of IKKε overexpression. Loss of IKKε function, meanwhile, had a positive effect on DIAP1 expression, resulting in a threefold increase. This set of findings led Oshima et al. to propose that IKKε and DIAP1 play opposing roles in regulating F actin-driven cellular morphogenetic events at the periphery of epithelial cells. Interestingly, however, the role of DIAP1, which is primarily known as a regulator of programmed cell death, appears to be independent of its anti-apoptotic function. “The IKK family of protein kinases is known to include regulators of immune response, so we were very surprised to find that IKKε has a completely different role in F-actin-dependent cellular morphogenesis,” admits Hayashi. “We were even more surprised to find that IKKε functions as a negative regulator of the non-apoptotic functions of DIAP1 and caspase. Since the IKKε-IAP pathway seems to be present in vertebrates, this finding might provide a clue to investigate evolutionarily conserved roles of non-apoptotic functions of caspases.” |
|||||||||
|
|||||||||
|
|||||||||
 |
|||||||||
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |