| Taking sides: Novel pathway establishes embryonic cell polarity in C. elegans |
 |
 |

August 28, 2006 – Embryos of the roundworm C. elegans develop distinct polarity even at the one-cell stage. Apparently triggered by cues linked to the sperm entry point at fertilization, this initial polarization helps set up the highly stereotyped and well-characterized sequence of cell divisions and differentiative events that ultimately results in a viable worm larva. The roots of this anterior-posterior cellular polarity have been traced to the asymmetric distribution of PAR-family proteins at the cortical periphery of the cell, in which PAR-3 and -6 localize to the anterior cortex along with their co-factor PKC-3, while PAR-2 accumulates at the cell’s posterior margin. But the means by which these proteins are conveyed to their appropriate destinations following a presumed signal from the sperm-contributed centrosome has never been worked out, although it is thought to involve the conveyance of the anterior PAR complex by the actomyosin cytoskeleton.
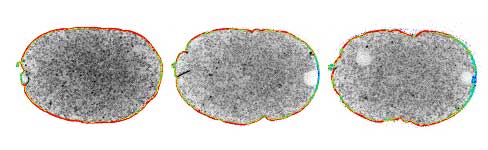 |
Local exclusion of ECT-2/RhoGEF from the posterior cell cortex leads to the establishment of anterior-posterior polarity in C. elegans embryos. Cortical ECT-2 visualized with GFP is pseudocolored. Higher and lower amounts of ECT-2 are represented by red and blue, respectively. Three sequential images are shown (posterior to the right). |
Now, Fumio Motegi and Asako Sugimoto of the Laboratory for Developmental Genomics (Asako Sugimoto; Team Leader) have followed the trail several steps farther upstream, elucidating the molecular pathway that goes a long way toward bridging the gap between the sperm entry site cue and the asymmetric allocation of PAR factors. In a study published in Nature Cell Biology, Motegi and Sugimoto have showed that two small GTPases, RHO-1 and CDC-42, and their potential regulator – ECT-2/RhoGEF – act in series to establish polarity in the one-cell roundworm embryo.
The Rho family of GTPases are known to participate in the regulation of the actin cytoskeleton, and as cytoskeletal activity had been implicated as a possible mode of transport for PAR proteins during cell polarization in C. elegans, the team examined the consequences of the loss of function of two Rho-family genes, cdc-42 and rho-1, and the gene for a putative RHO-1 activator, ect-2. RNAi suppression of the activity of these genes revealed an interesting disparity in the phenotypes. For all three factors, RNAi led to differing degrees of compromise of the posterior migration of the posterior mitotic spindle pole.cdc-42 loss-of-function cells showed normal initial polarization at the cell cortex, with ruffling at the anterior edge and smoothening at theposterior, but in both ect-2 and rho-1 RNAi cells, this effect was lost.
This preliminary finding led them next to look for possible relationships between these three factors and PAR localization. Developing a method for labeling and tracking the movement of PAR and cytoskeletal proteins using fluorescent tags, they determined that the polarization process is a two-stage affair. Phase I is characterized by the flow of cytoskeletal elements to the anterior cortex, whereas in phase II the newly established anterior cortical domain appears to undergo structural reorganization. Watching the effects of the loss of function of the three proteins, Motegi and Sugimoto found that when ect-2 or rho-1 function was interfered with, PAR-6 failed to localize to the anterior, while in cdc-42(RNAi) embryos, PAR-6 localized correctly in phase I, only to disappear in phase II. Similarly differential effects were observed in the behavior of the actomyosin cytoskeleton in the RNAi embryos, which led Motegi and Sugimoto to conclude that RHO-1 and its putative GEF, ECT-2, work in phase I to establish the anterior-posterior polarity of the cell, while CDC-42 functions in phase II to consolidate and maintain that polarization.
Experiments using fluorescent-tagged transgenes for ect-2, rho-1 and cdc-42 under the control of a germline-specific promoter enabled the direct monitoring of these factors. Analysis of the three proteins yielded observations that tended to confirm the predictions of the team’s working model, and revealed the dynamics and timing of their localization at new levels of detail. Prior to polarization, ECT-2 is uniformly distributed to the entire cortical region, but, perhaps in response to a molecular signal from the centrosome, ECT-2 gradually becomes depleted from the posterior cortex. It appears that this anterior concentration of ECT-2 then leads to the anterior accumulation of RHO-1. This prompts the anterior flow of actin cytoskeletal components, which is thought to be involved in conducting CDC-42 to localize in the same region enabling it to interact with both actomyosin and the anterior PAR complex.
“This study helps to explain some of the mechanisms underlying the earliest events in the A-P patterning of the roundworm embryo,” says Sugimoto. “It will be interesting to find out whether the establishment of polarity by the sequential action of Rho and Cdc42 has been conserved in other organisms as well.”
|


