| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
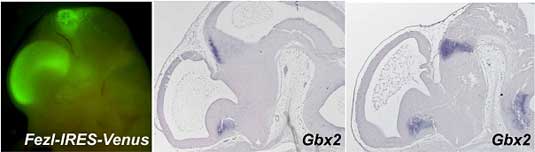
Now, in a study published in the journal Development, Tsutomu Hirata and Masato Nakazawa and colleagues in the Laboratory for Vertebrate Axis Formation (Masahiko Hibi; Team Leader) identify a pair of genes that work together to set up the initial subdivisions of the mouse diencephalon. Hirata and Nakazawa, working with researchers from a number of other CDB labs, describe how the zinc-finger genes Fez and Fezl cooperate to delineate and maintain forebrain patterning by repressing caudal specification in the rostral forebrain.
The strategy paid off, as Fez/Fezl double homozygous mice showed severe caudal forebrain defects, including the loss of the prethalamus and ZLI, and a significantly reduced thalamus (the pretectum, however, was intact). The Hibi team’s analyses of genes normally expressed in diencephalic regions showed that many genes directly or indirectly involved in the regionalization patterning of the forebrain were affected by the double homozygous mutation, suggesting that Fez and Fezl work together to regulate rostro-caudal patterning. Interestingly, in contrast to the deleterious effects on prethalamus and ZLI, the double homozygous phenotype showed a rostral expansion of the caudal regions of the diencephalon. Where the expression of genes normally associated with the more rostral prethalamus was lost, that of typically caudal markers was found to have shifted to the anterior, even from the earliest stages of neural patterning. For example, the expression of Irx1, a gene whose area of expression is ordinarily delimited at its rostral edge by the ZLI, was found to be shifted rostrally in the double mutant, bringing up the possibility that Fez and Fezl exert a repressive effect that normally prevents Irx1 (and other caudal diencephalic genes) from encroaching into the prethalamus. Tests using misexpressed Fez and Fezl tended to support the interpretation that these two genes in combination work to repress caudal diencephalon fate. Although morphologically abnormal, the diencephalon is not significantly reduced in size in the Fez/Fezl mutants, which led Hirata and Nakazawa to propose that these two genes function early in neural patterning to repress caudal gene expression in the rostral diencephalon, while at the same time promoting the establishment of the prethalamus. The reductive effects on the thalamus (which initially expands in these mutants) appear to be secondary to the loss of the ZLI, as this region serves as an inductive center for the thalamus, which it borders rostrally. It remains to be seen whether the repressive effects of Fez and Fezl on caudal fate determinants are direct or indirect, and how these genes themselves are regulated. Similarities to the genetic regulation of rostro-caudal regionalizaton of the avian brain, which has been extensively studied in chicken, may provide a basis for further study and, ultimately, a better understanding of how this complex pattern is laid down early in the development of the mammalian brain. |
|||||||||
|
|||||||||
 |
|||||||||
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |