| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Now, in an article published in Developmental Biology, Naoko Yoshida and colleagues from the Laboratory of Mammalian Molecular Embryology (Tony Perry; Team Leader) address this distinction in the first detailed study of the remodeling of sperm chromatin by metaphase II (mII) oocytes. During natural fertilization, the entry of a sperm induces a cell-cycle change known as meiotic progression, causing the oocyte to start behaving like an embryo. To measure the remodeling effects of mII (maternal), as opposed to post-fertilization (embryonic), cytoplasm on sperm chromatin, the team first eliminated the ability of sperm to induce meiotic progression. They confirmed that this had not otherwise compromised the biological potential of the inactive sperm by injecting them into oocytes and supplying an artificial signal to initiate development; the resulting embryos were able to develop to term, suggesting that the inactivation had not significantly altered the functional potential of the chromatin.
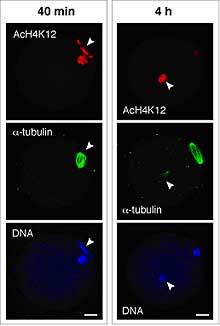
In mitotic cells, the level of histone acetylation influences gene expression, usually in the form of up-regulation, so it could play an important role in very early embryos. In the mII oocyte, paternal, but not maternal, chromatin acquired maternally-derived K12-acetylated H4 (AcH4-K12) independently of microtubule assembly and regardless of whether or not maternal chromatin was present. This state persisted without typical maturation-associated deacetylation, and was unaffected by the level of DNA (cytosine) methylation. In contrast, somatic cell nuclei underwent rapid H4 deacetylation; sperm and somatic chromatin even exhibited unequal AcH4-K12 dynamics when injected together into the same mII oocyte. This implies an extraordinary ability of the mII oocyte to discriminate between two types of exogenous chromatin and modify them in different ways. But how and why do mII oocytes achieve this discrimination? The inhibition of somatic histone deacetylation showed that histone acetyl transferase (HAT) activity was present in the oocytes together with histone deacetylase (HDAC). From a series of experiments, Yoshida and colleagues inferred that mammalian oocytes are able to specify the histone acetylation status of given nuclei by differentially targeting HAT and HDAC activities. However, the team also found that asymmetric H4 acetylation of the two parental chromatin sets during and immediately after fertilization was dispensable for development when both were hyperacetylated, so the function of this differential targeting remains enigmatic. |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |