| Researchers snare novel role for protein family |
 |
 |

November 10, 2006 – The complex system of nerves that surround the esophagus, stomach, and intestines consists of millions of sensory and motor neurons, information processing circuits and glial cells that are involved in transmitting and processing messages throughout this region. Known as the enteric nervous system (ENS), this network is comprised of neurons and supporting glia that line the intestinal wall and are derived from neural crest cells. These proliferating neural crest cells move through the developing intestine and migrate through the intestinal wall to subsequently differentiate into various neurons and supporter glial cells. The ensuing adult ENS plays a number of crucial roles within the enteric environment, including regulating intestinal motility and secretion, and controlling intestinal blood flow. Although a small number of genes have been identified as being involved in ENS development, they are insufficient to explain the complex processes involved in forming the ENS. Molecular mechanisms underlying neurite extension and ENS precursor migration, in particular, remain poorly understood.
Now, in an article published in the October issue of Developmental Biology, Hideki Enomoto (Team Leader; Laboratory for Neuronal Differentiation and Regeneration) and colleagues from the Washington University School of Medicine identify a number of genes that are highly expressed in the mouse ENS. In attempting to investigate these genes in more detail, Enomoto and colleagues found that members of the SNARE protein family play an important role in neurite extension and ENS precursor migration. Although SNARE proteins were known to function in mature synapses, this is the first report to show these proteins being involved in such an early stage of development as the formation of the ENS.
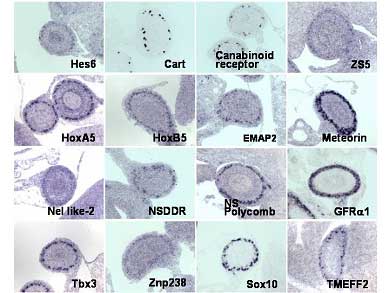 |
| The expression patterns of the genes identified by micro array analysis in ENS of E13.5 mouse embryo |
In an attempt to shed light on the genes involved in ENS development Enomoto and colleagues first compared gene expression in wild-type and aganglionic bowels from embryonic and newborn mice. This involved using a combination of DNA microarray analysis and quantitative real-time polymerase chain reaction (qRT-PCR). The results of this analysis revealed 83 genes that were expressed two-fold higher in the wild-type bowel than in the aganglionic bowel. The authors then selected 42 of these 83 genes for in-situ hybridization, of which 39 were found to be expressed in the ENS. This analysis also revealed a further 9 genes that had higher expression levels in the aganglionic bowel compared to wild-type mice, suggesting to the authors that intestinal innervation may influence gene expression in adjacent cells, such as the intestinal epithelium.
The majority of genes identified in this study all play an important role in synaptic function, such as Synaptotagmin 1, involved in fast neurotransmitter release, and Reticulon 1, which has a role in regulating exocytosis. Of particular interest to the authors, however, was the fact that these genes were expressed at E.14 – a stage of development in which the ENS is not yet required for survival. This prompted Enomoto and colleagues to put forward a scenario in which SNARE proteins are involved in the developing ENS. Armed with the knowledge that the SNARE family of proteins acts in membrane fusing of synaptic vesicles, the research team set out to examine the possibility that SNARE-mediated vesicle fusion is essential for both neurite extension and ENS precursor migration. In attempting to examine this issue in more detail, the authors subjected cultured embryonic bowel to a chemical inhibitor that blocked certain SNARE proteins, resulting in delayed neural-crest-derived cell migration and reduced neurite growth. Based on these findings Enomoto et al. concluded that the SNARE family of proteins is not only involved in synaptic function, but also plays an important role in ENS development.
In addition to successfully identifying a number of genes expressed in the enteric nervous system, this is the first study to show that SNARE proteins have an important role in ENS development. The authors hope that their current findings, together with their ongoing analysis of genes of interest, will provide a fresh perspective on irregularities in the ENS developmental process. Enomoto commented that “Developmental anomalies in the enteric nervous system are a direct cause of impaired bowel function in the form of Hirschsprung’s disease. We hope that the results of this study provide a basis for further research findings that could help in curing such diseases.”
|

