| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Now, in an article published in the journal Development, Makoto Ikeya and colleagues in the Laboratory for Organogenesis and Neurogenesis (Yoshiki Sasai; Group Director) report a gene, Cv2, which acts as a local enhancer of BMP signaling in mouse development. Named for its homology to the Drosophila gene crossveinless 2, which enhances BMP activity in the formation of cross-veins in the fly wing, mouse Cv2 works to amplify BMP signaling in the development of a number of tissue and organ systems, including bone, cartilage, kidney and eye.
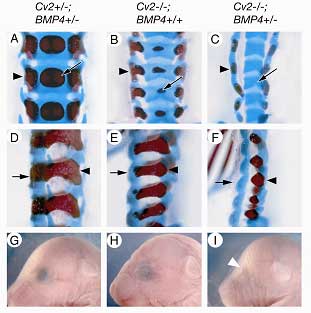
The pattern of these defects suggested that the loss of Cv2 prevents the differentiation of precursor cells derived from the sclerotome, a subset of cells that migrates out from the somites to give rise to the vertebrae. But analysis of gene expression by RT-PCR in tissues from the embryonic trunk indicated that the effect was not due to a simple, generalized reduction in BMP gene expression. Tests performed in cultured cells pointed instead to a role for Cv2 upstream of or at the level of the BMP molecular receptor. Interested in the nature of the interaction between Cv2 and BMP signaling, Ikeya next generated a series of mutants engineered to full or partial deletions of both genes, and found that in mice carrying a null mutation for Cv2, the loss of a single copy of the gene BMP4 significantly intensified the defects in both vertebral and eye development, suggesting a cooperative relationship between the genes. BMPs are known to be required for renal development as well, so the group next looked at the kidneys in the Cv2 mutants, and found that they were smaller and contained fewer renal glomeruli than normal. Taken together with the fact that the kidney mesenchyme normally strongly expresses Cv2, these findings pointed to an essential role for the gene in kidney development. On testing the effect of the loss of function of both Cv2 and the structurally similar kielin-chordin related protein (Kcp) on nephrogenesis, Ikeya et al found that the phenotype was more severe in the double mutant, indicating that the genes work together in the regulation of kidney development. Interestingly, the skeletal defects were unaltered in the Cv2/Kcp mutants.
|
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |