| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Now, in a pair of reports published in the same issue of the journal Biology of Reproduction, Manami Amanai and colleagues in the Laboratory of Mammalian Molecular Embryology (Tony Perry; Team Leader) describe new applications for, and insights into siRNAs and miRNAs in mammalian fertilization and early embryonic development. One of the papers describes what is perhaps the first close investigation into the role of miRNAs in the earliest stages of mammalian development, while the other reports technical advances in the use of siRNAs to silence genes in the oocyte and during and after fertilization.
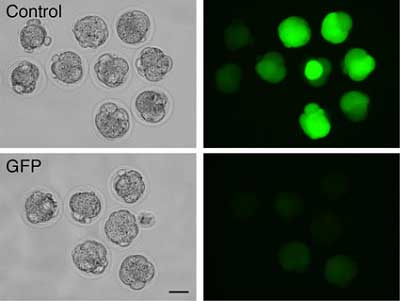
In contrast, nuclear transfer resulted in a significant change in the level of one of the 10 miRNAs tested, although enucleation itself had no effect on the profile; the change was therefore likely to have come during the deposition of the donor nucleus. This alteration of the miRNA landscape of mII oocytes by the incoming nucleus might be functionally significant, since embryonic development following nuclear transfer is so poor. In the second study, Amanai and colleagues made a proof-of-principle demonstration of the effectiveness of gene silencing by siRNAs in mII oocytes. The factor responsible for holding oocytes at mII, Emi2, was identified by the Perry lab in a previous report, but the roles of this and related cell cycle regulators in early embryogenesis were unknown, highlighting the need for new tools to study this fundamentally important process. Although siRNAs have been widely employed to dissect gene function - including gene function in mammalian embryos - they have not been characterized in mII oocytes. The team sought to test whether injected siRNAs could similarly be applied to the inhibition of regulatory factors at the first mitotic cell division following fertilization. The team injected siRNAs targeting cell cycle regulators expressed in the mII oocyte, and confirmed they brought about reductions in levels of mRNAs and corresponding proteins. They next checked whether RNAi initiated at the time of fertilization had an effect on the embryo in the earliest stages of its development, and determined that siRNA injected at the time of fertilization (i.e., sperm injection) can indeed knock down transcripts for both native genes and transgenes, with measurable effects up to the blastocyst stage. The approach also succeeded in nuclear transfer; co-injection of GFP-expressing donor cell nuclei with siRNA for GFP mRNA, produced resultant embryos which failed to glow (see Figure). This demonstrates for the first time the specific regulation of gene expression brought about during the nuclear transfer procedure. Since this regulation may be reversible, the approach promises one day to enhance reprogramming and the development of nuclear transfer clones by eliminating detrimental proteins. To demonstrate the workability of their technique, the team next used RNAi to examine any role in the first mitotic cell division played by the cell cycle regulators Cdc20, Emi1 and Emi2. By co-injecting sperm with siRNAs for any one of the three, they found that whilst Emi1 and Emi2 were apparently dispensable, Cdc20 depletion caused one-cell embryos to fail to cleave. Time will tell whether siRNA-mediated RNAi in mII oocytes - mII RNAi - will be of broad use, because some types of mRNA target seem more amenable to it than others. But its relative ease and specificity make it a promising new tool with which to study a difficult system.
|
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |