| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Studying the comparatively simple olfactory network of the fruit fly, Drosophila melanogaster, Keita Endo and colleagues in the Laboratory for Neural Network Development (Chihiro Hama; Team Leader) discovered a new role for an important molecular pathway in organizing this neuronal circuitry. In an advance online publication in Nature Neuroscience, Endo (now at the JST Institute for Bioinformatics Research and Development; Tokyo, Japan) shows that the differentiation of olfactory neurons deriving from the same precursor varies depending on the activity level of the Notch signaling pathway.
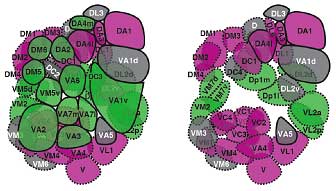
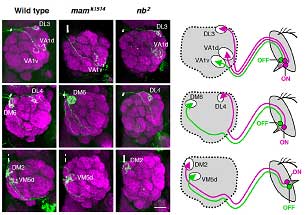
Endo et al. conducted a screen for mutations that disturbed the normal axonal patterning of olfactory receptor neurons in the fly, and identified a phenotype in which the axonal projection of a pair of neurons was disrupted. In this mutant, the neurons, which normally target separate glomeruli, both projected axons to the same destination. They identified the gene responsible as an allele of mastermind (mam—the new allele was christened mamk1514), which encodes a nuclear protein involved in the Notch signaling pathway. Notch signaling functions in numerous forms of cell differentiation, translating signals from the cell’s exterior via a Notch receptor to the nucleus, where it influences gene expression. Knowing that Notch signaling had been shown previously to work in asymmetric cell divisions (in which two daughter cells of different character are generated from a single precursor), via the accumulation of the Notch antagonist Numb (nb), the team next looked at a numb mutant, and found that the phenotype was the precise opposite of the unilateral axonal projection seen in the mamk1514 fly. To develop a more comprehensive picture of the role of these Notch factors, they first surveyed the organization of glomerular clusters in wild-type flies, and identified 52 glomeruli grouped in 24 clusters, each of which receives axonal projections from a single clonal cluster of olfactory receptor neurons. Their map showed that all ORNs housed within the same sensillum (a sense organ comprising one to four ORNs) are clones, deriving from the same precursor. To identify which glomeruli in each cluster receives projections from ORNs in the mam and nb clones, flies were engineered to contain small mutant clones of cells that included ORNs. This experiment revealed that each glomerular cluster received projections from a combination of ORNs present in mam clones and nb clones. This was the first evidence that the differentiation of olfactory neuronal clusters could be determined by the activation or absence of Notch signaling. An examination of the Notch state across all parts of the olfactory system show that this effect functions globally—asymmetries in Notch signaling in olfactory receptor neurons within a clonal cluster determine the patterns of axonal projections to specific glomeruli throughout the system.
This clean segregation of olfactory receptor expression and axonal projection of clonal ORNs based on their Notch activity state represents a major new insight into the understanding of an important and highly complex sensory system. “What we have revealed here is only one element of the mechanisms used for generating a diverse array of ORNs derived from sensory organ precursors,” notes Hama. “It raises a number of intriguing questions concerning the genes downstream of Notch and Notch-independent mechanisms for ORN diversification. We are also curious as to whether the domain organization of glomeruli created by Notch-ON and –OFF classes of ORNs represents any functional or behavioral relevance in olfaction.” |
|||||||
|
|||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |