| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Recent work from the Laboratory for Cell Fate Decision (Hitoshi Sawa; Team Leader) is set to change that. In an article published in Developmental Cell, Kota Mizumoto, a grad student in the Sawa lab proposes a general model of how cortical WRM-1 interacts with other factors in the Wnt pathway, such as the APC homolog APR-1, to regulate the localization of WRM-1 in the nucleus, where it would otherwise work as a transcriptional activator.
The first step toward answering that question came when the team causing WRM-1 to localize throughout the entire cortex, rather than only the anterior, in the T cell lineage that appears in postembryonic development. This strategy involved forcing the expression of a fusion protein (WRM-1::GFP::CAAX); in this construct, CAAX effectively tethers WRM-1 to the cortex, while GFP aids in visualization. Worms engineered in this way showed the same phenotype as mutants in which wrm-1 function is lost (i.e., on division, both daughter cells shared a hypodermal cell fate, in contrast to one hypodermal and one neural cell in wildtype) showing that the defect was an outcome of the uniform localization. Mizumoto further found that LIT-1, the C. elegans homolog of MAPK, also localized symmetrically at the cell surface. This is significant, as MAPK has also been shown to play a mediating role in asymmetric cell division.
“It appears that somehow APR-1 enables WRM-1 at the anterior cortex to prevent the accumulation of WRM-1 in the anterior nucleus,” says Mizumoto. “When we looked at the movement of WRM-1 out of the nucleus, it appeared that more WRM-1 remains in the nuclei as a result of RNAi knockdown of apr-1, which we think has to do with its role as a mediator of nuclear export.” PRY-1, the homolog of Axin, another factor in the destruction complex, also localized asymmetrically to the anterior cortex as did APR-1, suggesting that these may be components of a complex along with WRM-1. This asymmetry failed to occur in Wnt mutants, indicating that the effect is likely regulated by Wnt signaling. “We still don’t have the molecular mechanism that explains the precise way in which APR-1 mediates the inhibition of WRM-1 accumulation in the anterior nucleus by WRM-1 at the anterior cortex,” Mizumoto admits, “but we do have a reasonable model of how it might work through the asymmetric export of WRM-1 from the nucleus. But as apr-1 also appears to be required for the nuclear localization of WRM-1 in other cell types, there may still be some surprises ahead.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |
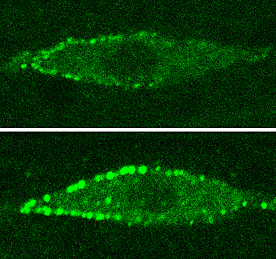
 Given the central role of WRM-1 in Wnt signaling, Mizumoto next looked at APR-1, the homolog of APC, a member of a group of factors that form what is known as the “destruction complex,” which marks β-catenin for destruction in the absence of Wnt. In worms in which apr-1 was knocked down by RNAi, the phenotype was opposite that of the WRM-1::GFP::CAAX mutant; both daughters became neural, not hypodermal. The defect was a mirror image of that of other mutants in which the Wnt/MAPK pathway is upregulated, suggesting that apr-1 negatively regulates this pathway. Inhibition of apr-1 also disturbed the pattern of asymmetric WRM-1 nuclear localization, which normally causes it to localize in the posterior nucleus from about the telophase stage of cell division. In the apr-1 knockdown phenotype, the distribution was symmetric to both nuclei.
Given the central role of WRM-1 in Wnt signaling, Mizumoto next looked at APR-1, the homolog of APC, a member of a group of factors that form what is known as the “destruction complex,” which marks β-catenin for destruction in the absence of Wnt. In worms in which apr-1 was knocked down by RNAi, the phenotype was opposite that of the WRM-1::GFP::CAAX mutant; both daughters became neural, not hypodermal. The defect was a mirror image of that of other mutants in which the Wnt/MAPK pathway is upregulated, suggesting that apr-1 negatively regulates this pathway. Inhibition of apr-1 also disturbed the pattern of asymmetric WRM-1 nuclear localization, which normally causes it to localize in the posterior nucleus from about the telophase stage of cell division. In the apr-1 knockdown phenotype, the distribution was symmetric to both nuclei.