| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
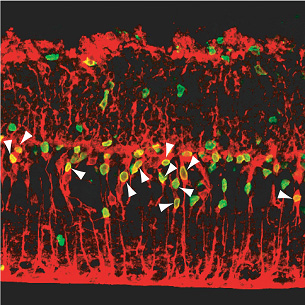
“It’s still very early days,” admits Osakada, “but as my background is in pharmacology, it’s interesting to think that it may be possible one day to develop an injectable small molecule of similar function that would boost this in-built regenerative mechanism, rather than relying solely on the more frequently proposed transplantation of cells grown in culture.” The study began as a follow-up on previous findings from the Takahashi lab, which showed for the first time that non-neuronal glial cells from a mammalian (rat) retina are able to backtrack to a less mature stage on their differentiation pathway following retinal damage. This regenerative capacity was extremely limited, however, and the hunt was on to identify factors able to improve it. After testing of various candidates, Osakada et al decided to look at the effect of Wnt signaling on retinal neuron regeneration. Wnt factors are prevalent in aspects of development ranging from limb development to axon guidance, and are known to control the self-renewal of certain forms of stem cells as well, but their study of Wnt function in the adult central nervous system has been hampered by the fact that many mutations affecting this signaling pathway result in embryonic lethality. By developing a system for administering Wnt directly into an in vitro model of retinal damage, detecting the number of glial cells that re-entered the cell cycle and tracing their progeny, the Takahashi lab was able to show that Wnt3a treatment significantly increased the level of proliferation of neuronal progenitors from the de-differentiated cells. Tracking the fates of these Müller glia-derived progenitor cells, Osakada et al. determined that these cells migrate into the outer nuclear layer of the retina, where they differentiated into rod photoreceptors, capable of detecting light. They further verified the involvement of the canonical Wnt pathway, in which Wnt activation protects cytoplasmic β-catenin from degradation enabling its accumulation in the nucleus and function as a regulator of gene transcription. In animals in which retinal injury had been induced, they found that trauma caused an increase in the nuclear accumulation of β-catenin. Blockage of Wnt signaling by the inhibitor Dkk1 led to a decrease in the number of post-injury retinal progenitors and the regenerative effect of Wnt3a treatment could be mimicked by the administration of a GSK-3b inhibitor (GSK-3b is normally inhibited by Wnt activation). Retinal degeneration, such as occurs in retinitis pigmentosa, is a deteriorative condition in which the rod and cone cells of the eye gradually die, leading to vision impairment and eventual blindness. The lab was understandably interested in finding out whether the Wnt-induced regeneration they had observed in an in vitro retinal injury model would be seen in a mouse genetic model of degeneration as well. Using retinal explants from rd mutant mice, which exhibit dramatic retinal degeneration from a very early age, they found that, as in the injury model, Wnt3a boosted cell proliferation and that the resultant progenitors could then be steered to differentiate into photoreceptor cells. Osakada notes, “While this work was done in vitro, the eye itself is an attractive target for this type of therapeutic concept, as it is relatively isolated from the rest of the nervous system and you can control the dose and area of delivery.” This report represents an important step forward in the understanding of the molecular mechanisms that underlie the innate ability of the mammalian retina to respond to damage, and points to one possible approach into the pharmacological remedy of retinal degeneration. “Much remains to be done before we can start thinking about actual therapeutic applications,” cautions Masayo Takahashi, who heads the lab where the study was done. “These findings still need to be confirmed using human cells and, as a protein, Wnt is too unstable to be used as a treatment, but we are nonetheless very excited to have been able to show for the first time that it is possible to promote the regeneration of photoreceptive neurons in a mammalian model of retinal damage.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |