| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
This question has now been addressed in a study by Masaki Fujita and others in the Laboratory for Cell Fate Decision (Hitoshi Sawa; Team Leader), which looked at how a tightly maintained balance of the activity of cell cycle regulators keeps such cells from differentiating in the roundworm, C. elegans. In an article published in the open access journal PLoS One, the team describes how the interplay between the worm homologs of a triad of cell cycle regulators—cyclin E, CDK2 and CKI—simultaneously prevents differentiation and division in uncommitted, quiescent cells.
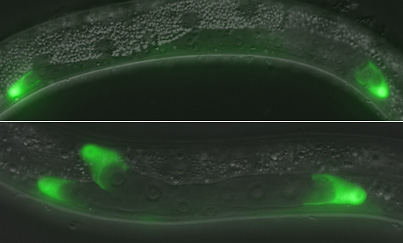
Cyclin E is known to function cooperatively with a second cell-cycle protein, CDK2, in other organisms, so Fujita et al. next examined the effects of its loss of function by inhibiting the roundworm gene using RNAi. As with the cye-1 mutants, the cdk-2 knockdown worms showed the extra DTC phenotype, in a manner suggesting that the two factors act in partnership. Cell-cycle regulators such as cyclins and cyclin-dependent kinases are typically kept in check by factors known as CKIs. On analyzing the patterns of expression of cye-1 and the roundworm CKI, cki-1, in worms in which the protein products of these genes had been engineered to fluoresce green, the Sawa team found that they were expressed asymmetrically, with cye-1 levels higher in the Z1.ap and Z4.pa cells (which are normally quiescent) and cki-1 expression stronger in the Z1.aa and Z4.pp cells that normally differentiate into DTCs. Using a temperature-sensitive mutation of wrm-1 (roundworm β-catenin) to allow them to initiate its loss of activity after the division of the Z1/Z4 cells, they found that this unequal distribution is under the control of the Wnt/MAPK pathway, as is the case in a number of other asymmetric cell divisions in C. elegans. Given the higher levels of cki-1 in the quiescent sisters of the DTCs, the lab next looked at a possible interaction between this gene and the partnership of cye-1 and cdk2. In worms in which cki-1 alone had been knocked down, the majority of animals showed extra cell divisions, but when this interference was combined with either cye-1 mutation or cdk-2 RNAi, no such phenotype occurred, suggesting that cki-1 inhibits the proliferation of the Z1.ap and Z4.pa cells, preventing cye-1/cdk-2 from triggering cell division but leaving their activity high enough to prevent terminal differentiation. A similar mechanism appears to be at work in at least one other cell lineage exhibiting asymmetric cell divisions, as usually quiescent seam cells in cye-1 mutant worms adopted an abnormal syncytial fate, indicating that cye-1 is needed to repress terminal differentiation in multiple quiescent cell types. “Asmany stem cells are quiescent, the cell cycle regulators we identified as functioning in this context may also serve to maintain the undifferentiated state of stem cells in other organisms,” notes Sawa, highlighting the potential importance of this new role for cyclins, CDKs and their inhibitors in maintaining quiescence.
|
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |