| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
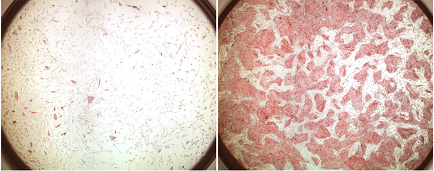
The Sasai group has developed a number of techniques for inducing the differentiation of mouse ES cells into specific neuronal fates to date, some of which, such as the generation of dopaminergic neurons (which are deficient in Parkinson’s disease) have been reproduced using human ES cells as well. But the susceptibility of hES cells to cell death when subjected to stressful culture conditions has remained a major obstacle to fulfilling their clinical promise. These cells are especially sensitive to dissociation, in which digestive enzymes are used to separate individual cells in order to expand a single population into multiple colonies. While this is standard protocol for mouse ES cells, the dissociation of human ES cells it causes 99% attrition due to cell death within two days, which has mean that hES researchers have been forced to resort to other, more finicky methods. By developing a means of preventing such massive die-offs, Watanabe, now at the California Institute of Technology, has put mouse ES cell culture techniques within reach of the human embryonic stem cell research community. The study began with indications that an atypical form of cell death observed in specific cell types, such as motoneurons, might be linked to the activity of ROCK (Rho-associated coiled coil kinase). On looking its expression in dissociated hES cells, he found that ROCK became activated soon after trypsinization. On pretreatment with the ROCK inhibitor Y-27632, isolated hES cells showed a nearly thirty-fold increase in the efficiency at which they could be cloned into new colonies. This boost in survival and proliferation was seen in other culture methods as well, and had no detectable effect on the cells’ pluripotency or self-renewal. ROCK inhibition also considerably facilitated the selective cloning of hES cells after gene transfer. The introduction of exogenous genes into the genome of hES cells is a notoriously difficult procedure, with a success rate of less than one in a thousand tries, meaning that transgenesis experiments performed to date have required enormous quantities of cells. But the dramatic reduction of hES cell death by treatment with Y-27632 or other ROCK inhibitors now makes it possible to conduct such studies on a single 10-cm plate. In an exciting proof-of-concept demonstration, Watanabe et al. further showed that ROCK inhibition can be used to enable the neural differentiation of hES cells. The Sasai group had previously developed a method known as SFEB (for Serum-free Floating culture of Embryoid Body-like aggregates) for inducing the differentiation of mouse ES cells into cells expressing genes characteristic of embryonic telencephalon, but as this required the dissociation and suspension of the cells, the technique was impracticable for use in human ES cells.But the improved robustness of ROCK-inhibited cells to such unfavorable culture conditions made it possible for even hES cells to proliferate in SFEB culture, and the group found that after 35 days of culture in the presence of inhibitors of neural antagonists a full third of the cells had taken on a telencephalic precursor or neuronal fate.
“We look forward to sharing this method with collaborating labs and institutes around the country, in the hopes of accelerating the pace of biomedical research,” says Sasai. “And at the same time, we will be looking very closely at why it is only human ES cells that exhibit this peculiar form of cell death and the role that ROCK plays in that process.” |
|||||||
|
|||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |