| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
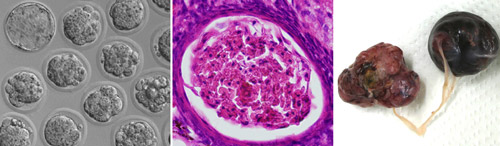
The transgenic lines behaved similarly to each other. Pups were born and developed normally at first. However, females exhibited very low fertility. Their oocytes underwent normal maturation, arriving at mII just like those of their non-transgenic littermates. But unlike normal oocytes, the ones from transgenic mice then immediately progressed to anaphase II and beyond, as if they had been fertilized, often developing to the blastocyst stage in vitro. These findings indicate the exquisite specificity of PLCZ1, which enables it to induce parthenogenetic exit from meiosis II (it is even specific to a particular stage of a particular cell-cycle) in otherwise healthy mice. “This is the first demonstration of ovarian teratoma formation in mice whose oocytes complete meiotic maturation,” according to Perry. The work doesn't categorically address whether additional sperm proteins are normally required to lower the threshold of the PLCZ1 signal, because the amount of transgenic PLCZ1 in the oocytes was too small to measure. But the group were able to show that the PLCZ1 does act directly. When they transferred Plcz1 transgenic cumulus cell nuclei into wild-type oocytes, the oocytes frequently became activated and could develop to the blastocyst stage. It remains to be seen whether the PLCZ1-expressing cells of these mice support full development as if they were 'somatic sperm'. Although Plcz1 transgenic mice initially appeared normal and healthy, females developed abdominal swellings caused by ovarian tumors. The frequency of tumour development in females was high (~70% after 6 months) but tumors were never found in transgenic males, corroborating the specificity of the phenotype. The simplest explanation for the tumors is that PLCZ1-induced parthenogenesis occasionally occurred in mature oocytes that failed to be ovulated, and that the resultant trapped parthenogenotes subsequently underwent unchecked ovarian growth to yield the tumors. Questions, however, remain. Most tumors, although occurring in a hemizygous background, are apparently hemizygous, yet the 'tumor-from-parthenogenote' model doesn't readily explain how. In addition, the parthenogenotes often underwent uterine implantation, but never induced uterine tumorigenesis. It is unclear why ovarian, but not uterine, sites should foster tumour formation. The tumors in some cases accounted for a large proportion of the total body mass, so given their embryonic etiology it is unclear why metastasis was never observed. Several of the PLCZ1-expressing transgenic mice developed ataxia, a presentation for a subset of clinical ovarian cancer patients. However, the group did not find evidence for mutations in the PLCZ1 gene in human breast epithelial, ovarian epithelial or benign ovarian germline tumors. Nevertheless, PLCZ1-expressing transgenic mice provide a tractable model for the study of ovarian tumor development, and indicate that PLCZ1 provides an intriguing link between fertilization and tumorigenesis.
|
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |