| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
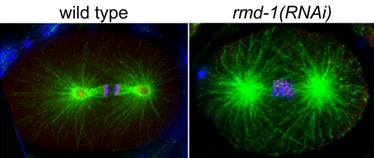
Kumiko Oishi, a research scientist working in the Laboratory of Cell Fate Decision (Hitoshi Sawa; Team Leader) has now added an important new piece to the puzzle, with the identification of rmd-1, a novel C. elegans gene that localizes to spindle microtubules and attachment sites at spindle poles. Working in collaboration with a team at the Keio University School of Medicine, Oishi showed that this microtubule-associated protein is required for chromosome segregation, possibly through an interaction with aurora B kinase, another factor believed to be involved in the recognition and correction of abnormal chromosome-cytoskeleton attachments. The Sawa team first identified the rmd-1 phenotype in a screen for mutants lacking a specific cell type known as phasmid socket cells, and pinpointed the genetic defect to a single point mutation. The gene encodes a hitherto unknown protein, but one which, interestingly, has possible homologs in frog, zebrafish, mouse and human. Its pattern of expression shows a high level of co-localization with tubulin (a microtubule marker) during cytokinesis. She next studied the loss of function phenotype of rmd-1, using RNAi to knock the gene down. The rmd-1-inhibited embryos showed a high incidence of defects in chromosome segregation, and other aspects of cytokinesis, such as spindle orientation. A subsequent series of experiments showed that these defects were caused by the failure of the microtubules to attach properly to the chromosome, at sites called kinetochores. Specifically, chromosomes in rmd-1 (RNAi) embryos tended to form merotelic attachments, in which a single kinetochore becomes attached to microtubules from both spindle poles, rather than just one. The defects in chromosome segregation in rmd-1 (RNAi) embryos bore some resemblance to those seen in other organisms in mutants for aurora B, a kinase that destabilizes microtubules that have attached incorrectly to chromosomes. When Oishi conducted a pull-down assay to test for protein-protein associations, she found that RMD-1 indeed co-precipitates with the C. elegans homolog of aurora B. While the Sawa team further determined that RMD-1 plays an additional role in orienting the spindles at opposite poles, they were able to rule out the possibility that the rmd-1 (RNAi) causes ploidy problems by slowing microtubule growth, strengthening the case for a role in the aurora B-mediated pathway, which serves an important editorial function in maintaining proper chromosome segregation. This is of particular interest, as the evolutionarily conserved nature of rmd-1 suggests that its human counterpart may play a role in preventing aneuploidy, which is implicated in cancer and various congenital anomalies, in humans as well. Commenting on the discovery, Oishi says, “We hope that our findings will contribute to a fuller understanding of the evolutionarily conserved mechanism that maintains the fidelity of chromosome segregation. The discovery of the RMD protein family may also deepen our knowledge of microtubule-based processes.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |