| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
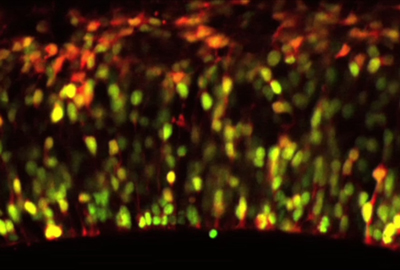
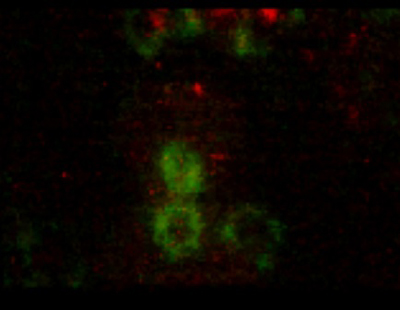
Daijiro Konno, Go Shioi and colleagues in the Laboratory for Cell Asymmetry (Fumio Matsuzaki; Group Director) have now shed light on the control of spindle orientation in neuroepithelial cells, and its function in maintaining the neuroprogenitor population. This work, published in Nature Cell Biology, identifies the gene LGN as a critical component for orienting the mitotic spindle so as to allow neuroepithelial cell division to proceed asymmetrically. Mitotic spindle orientation has been studied extensively in the fruit fly Drosophila melanogaster, revealing an elaborate network of genes that steers the spindles and links them to the cell cortex. Recent work from a number of labs has suggested that similar genetics may underlie this process in mouse neuroepithelium as well. Shioi and Konno began by creating a mutant mouse lacking the gene LGN, a murine counterpart to the Drosophila gene, Pins (Partner of Inscuteable), which participates in the cortical complex responsible for spindle orientation in the fly. Looking at dividing cells in the brain preparations from the LGN knockouts, they found that their orientations had become randomized, rather than occurring parallel to the epithelial surface as they do in wild type animals. A similar spindle misorientation phenotype could also be produced by overexpression of mInsc, the mouse ortholog of Inscuteable, a Drosophila gene that functions to rotate the mitotic spindle perpendicular to the epithelial surface. To determine whether such misorientation had an effect on daughter cell fate, the Matsuzaki group next examined the distribution of LGN mutant daughter cells expressing the gene Pax6, which normally marks apical progenitors. They found that, rather than remaining in the ventricular zone (the region nearest the apical aspect of the neuroepithelium, where cell division generally occurs), Pax6 cells were scattered more basally. This was also the case for cells overexpressing mInsc, suggesting linkage between the failure of the spindles to orient properly and the basal scattering of progenitors. Apical progenitor cells are normally polarized, extending processes called “feet” from both their apical and basal surfaces. In wild type, most progenitors divide in such as way that both daughters inherit part of the mother cell’s apical foot. But a third member of the group, Atsunori Shitamukai, found that in the orientation mutants, the apical foot was segregated to only one of the daughters at a much higher frequency. In both cultured brain slice preparations and in vivo, the progenitors produced fewer apical and more non-surface progenitors on division, and as it is the non-surface progenitors that primarily generate neurons, the rate of neurogenesis was unaffected despite this change in composition. Studies of flies bearing mutations for various genes associated with EGFR signaling showed that this pathway is required for both the apical constriction that creates the tracheal pit and for tracheal invagination to occur at the proper timing. In Egfr mutants, the onset of invagination was delayed and the apical constriction significantly impaired, and similar, though less pronounced, phenotypes were seen in mutants for an EGFR-activating ligand and a target factor that functions downstream of the EGF receptor. These mutants also exhibited defects in the cell arrangements responsible for controlling the location of the tracheal pit, where invagination occurs; in contrast to wildtype, mutants frequently developed ectopic or supernumerary tracheal pits.
“It is predicted that randomizing the orientation of cell divisions should affect epithelial morphogenesis and other phenomena, “ notes Matsuzaki. “So the finding that LGN knockout mice, in which the orientation is indeed random, are viable came as something of a surprise to us, and highlights the robustness in the face of perturbations of mammalian development.” |
|||
|
|||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |