| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
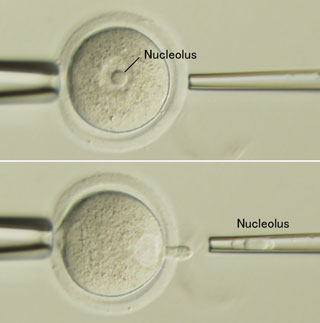
New work by Sugako Ogushi in the Laboratory for Mammalian Germ Cell Biology (Mitinori Saitou; Team Leader) now takes a major step toward answering that question with the demonstration that the oocyte nucleolus is essential to early embryonic development in mammals. This study, which was done in collaboration with scientists from Kobe University, Italy, and the Czech Republic and published in the journal Science, shows for the first time the indispensable role played by the maternal nucleolus, possibly as the source of materials needed to form the zygotic nucleolus in the first stages of embryogenesis. The nucleolus, which forms a part of the nucleus, is mainly responsible for assembling ribosomes, the protein factory organelles in which peptides are translated from mRNA. However, a fully-grown oocyte completely ceases transcription including ribosomal RNA synthesis. Its nucleolar structure apparently contains no DNA and shows highly compacted, monotonous morphology of uncertain composition and function. Although nuclear transfer generally involves the removal of the entire nucleus, Ogushi used a micromanipulation system to selectively pluck out only the nucleolus from oocytes taken from pigs and mice, a procedure called enucleolation. She found that, although these enucleolated oocytes were capable of normal maturation and could be activated parthenogenetically or by fertilization, the activated cells failed to develop nucleoli of their own. Interestingly, this defect could be rescued by reinjecting the maternal nucleolus into an enucleolated egg, but not by injection of nucleoli from somatic or ES cells. Following the development of enucleolated embryos, Ogushi found that pig embryos produced from oocytes lacking nucleoli failed to develop past the first few cleavages, although their viability was restored by reinjection of the missing maternal nucleolar material. Oocytes that were sham-operated by aspirating a small amount of nucleoplasm but leaving the nucleoli intact developed zygotic nucleoli, and did not suffer the same embryonic arrest, suggesting that the enucleolated embryos’ failure was not simply due to the trauma of micromanipulation. Suspecting that the maternal nucleolus might be the missing ingredient preventing SCNT embryos from developing normally, the team next tried injecting maternal nucleoli along with somatic cell nuclei into oocytes that had had their own nuclei removed. This nucleolar coinjection alone, however, was insufficient to rescue development, indicating that the maternal nucleolus is a necessary, but not sufficient, component for mammalian embryogenesis. It is likely that additional factors in the germinal vesicle (as the oocyte nucleus is called) are also required, as oocytes in which the germinal vesicle has disintegrated, releasing its contents into the cytoplasm, support development after nuclear transfer, while those in which the germinal vesicle has not broken down, do not. The discovery that nucleoli are inherited solely from the mother adds these structures to the list of organelles, such as mitochondria, that are passed on from only one of the parental lineages. It has been known for years that the early embryo relies on a stock of materials from the oocyte to drive development over the first few rounds of cell division. These new findings indicate that the nucleolus may be an important maternal contribution, needed by the embryo to build nucleoli of its own. “In this study, we showed that the nucleolar structure in a fully-grown oocyte is essential for early embryonic development, and that it contributes to the nucleolar structure of the paternal pronucleus, demonstrating that the nucleolus of a zygote is exclusively of maternal origin” says Ogushi. “Although we still don’t know its precise function and molecular components, we are now working to answer those questions. We hope these studies will lead to a better understanding of how a totipotent zygote is constructed.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |