| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
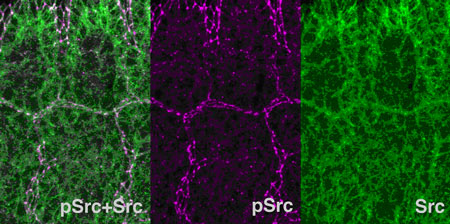
A study by Masayo Shindo and colleagues from the Laboratory for Morphogenetic Signaling (Shigeo Hayashi; Group Director) has taken a new tack by looking at the function of Src in epithelial morphogenesis in the fruit fly Drosophila melanogaster. Their report, published in Development, suggests that Src has the somewhat paradoxical dual function of suppressing cell adhesion by E-cadherin while simultaneously upregulating its expression. The study began with a gain of function screen for mutations producing altered epithelial morphology during tracheal development in the fly, which yielded the Src family members Src42A and Src64B, both of which caused defects in tracheal integrity on overexpression. Using antibodies to monitor the activity of these genes in vivo, the authors determined that these genes were upregulated in a range of epithelial tissues undergoing cell rearrangement. Focusing back on the embryonic tracheal system, the group next studied the effects of loss of Src42A function and found multiple defects involving a ramified structure known as the dorsal branch (a group of cells that gives rise to the trachea), including reductions in number of cells, and delays in branch extension and cell intercalation. Knowing of the link between Src and E-cadherin in other contexts, Shindo et al. re-examined the function of E-cadherin in tracheal development using the cadherin gene, shotgun (shg). Loss of function shg mutants showed disruptions in cellular attachments in tracheal branches, while its overexpression caused delays in dorsal branch elongation and cell rearrangements. Over activation of Src42A caused reduced tracheal cell adhesion phenotype, which was partially suppressed by co-expression of E-cadherin, suggesting that E-cadherin is a rate-limiting component of the tracheal cell adhesion under control of Src. They next used a technology known as fluorescence recovery after photobleaching (FRAP), in which a fluorescent-tagged sample is zapped with high intensity light causing it to lose its fluorescence (known as “bleaching”) and then watched to determine the rate of recovery, to study the effects of Src on adherens junctions, where cadherins normally accumulate. Looking at GFP-labeled α-catenin, an E-cadherin-binding molecule, as a measure of adherens junction dynamics, they made the interesting discovery. Entry of α-catenin-GFP into adherens junction was slowed down upon loss of Src function and increased upon gain of Src function. “We suspected that Src enhances turn over of α-catenin in adherens junctions,” says Hayashi, who heads the lab in which the study was done. One puzzling question remained. Although reduction of E-cadherin explains inhibition of cell adhesion by Src, it does not explain why activated Src permitted a larger amount of α-catenin to enter adherens junction. The hint was obtained when they investigated the expression of Armadillo (the Drosophila version of β-catenin, another binding partner of cadherin). They discovered that Src promoted Armadillo expression and its downstream target, E-cadherin. This led to the remarkable conclusion that, even as Src down-regulates the E-cadherin protein at the adherens junction, it upregulates E-cadherin transcription. This dual function explains increased turn over rate of cell adhesion molecules upon elevation of Src, making adherens junction more dynamic. “The dual function of Src we’ve discovered provides an answer to the long-standing question of why embryonic epithelial tissues are plastic enough to be able to adopt a variety of morphologies,” says Hayashi. “The mechanism also explains why Src-transformed cells not only detach from original tissues, but also re-settle frequently in a new position, an essential feature of malignant cancer metastasis.”
|
|||||||
|
|||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |