| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
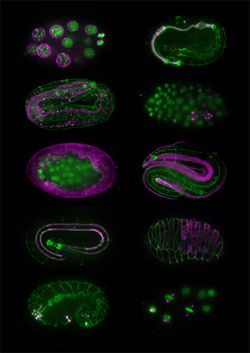
May 16, 2008 – Antibodies have become an indispensable component of the experimental repertory in many life sciences laboratories, used in such techniques as immunohistochemistry, Western blotting and immunoprecipitation. It remains a challenge, however, to isolate antibodies of precisely the right affinity for antigens called for under a given experimental design. A newly published method developed by Kazumasa Takeda and others in the Laboratory for Developmental Genomics (Asako Sugimoto; Team Leader) now looks set to lower the barrier to the creation of monoclonal antibodies to low-abundance antigens. In a proof-of-concept demonstration published in Genes to Cells, of this “antigen subtraction” approach, the team was able to isolate more than 30 monoclonal antibodies specific to structures in the C. elegans embryo, including P granules (specifically present in cells of the germline), muscle, pharynx and hypodermis, as well as previously unreported cellular structures. Importantly, this method can be applied to other species as well, which will allow researchers in other labs to develop new, highly specific antibody probes for cells and tissues at specific developmental stages.
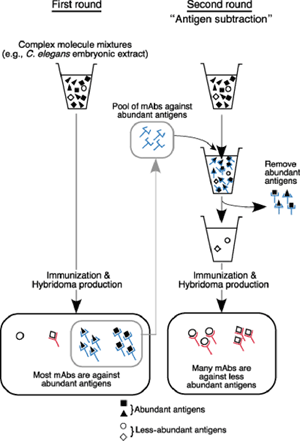
The project traces its roots back more than 20 years, to when Takeda was using monoclonal antibodies as part of a virus research project at Kyoto University. On reading a review on apoptosis and roundworm germline development, he wondered whether he might be able to apply the techniques he’d been using in virology to develop antibodies for specific C. elegans structures as well, and quietly began to experiment. In the late 1980s, he generated and screened more than 1,300 hybridomas (cancer cell engineered to produce a single species of antibody). “I had to be a bit careful when I was working on these,” admits Takeda, “as they weren’t really part of the research program of the lab I was working in at the time.” Despite his intensive initial efforts, Takeda was frustrated to find that many of his antibodies were not specific to any single structure in immunostaining, and tended to stain the entire C. elegans embryo. Reasoning that the solution would lie in finding a way to sift out non-specific antibodies, he devised a clever two-stage system in which first antibodies against abundant and non-specific antigens are pooled and then used to subtract out the corresponding antigens from the embryonic extracts. The mixture that remained after this subtraction should then be enriched with less abundant proteins, which could then be used for a second round of immunization, hopefully yielding targets capable of marking specific cell or tissue types. Takeda found that in the first round of immunization, only about 3% of monoclonal antibodies showed a specific pattern in immunostaining tests; the rest generally stained the entire embryo. But after subtraction, almost 50% produced signals in specific structures or cell types. Takeda had to discontinue this project for various reasons, but in 2003, after retiring from Kyoto University, Takeda emailed Sugimoto to ask her if she would be interested in his collection of hybridomas and monoclonal antibodies, which led to an offer to join her lab and resume his work on an official basis. Takeda and Chie Watanabe, a technician in the lab, then developed a collection of 35 monoclonal antibodies for a range of structures including P granules, body wall muscle, hypodermal cells, and pharynx. This antibody library has already been used to produce images by immunostaining of previously unknown histological and cellular structures, which can be viewed on the Sugimoto lab homepage: http://cdb.riken.jp/dge (click on the link for the KT mABs DB). The lab has already received more than 30 requests for antibodies from labs around the world, and is pleased to make them available to the global C. elegans research community. Within the lab, the antibodies have opened up new direction for research and are now being used in analyses of the structure and assembly mechanism of P granules. “The C. elegans research community has always been based on the open sharing of information and findings,” says Sugimoto. “So we have made the entire database accessible to everyone in the field, in the hopes of distributing these antibodies as widely as possible.”
|
|||||||
|
|||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |