| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
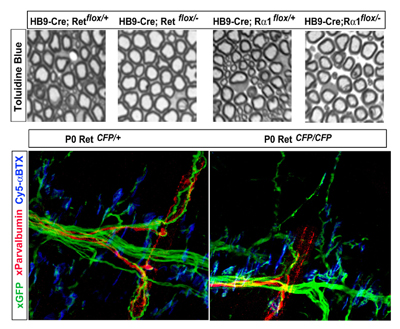
June 5, 2008 – Neurons are born with an appetite for molecular factors that regulate multiple aspects of their development and function, and in many cases are necessary for their very survival. One such neurotrophic factor, GDNF (glial cell-line derived neurotrophic factor), is known to play important roles in gene expression, targeting, survival, and synapse pruning in spinal motor neurons, which innervate skeletal muscles and convey efferent signals from the brain. Skeletal muscle comprises different kinds of muscle fibers – the extrafusal fibers that contract and extend to generate tension, and the intrafusal fibers, which are sheathed in sensory receptors that detect the speed and length of a muscle as it is stretched. Mice lacking GDNF or its receptors are known to suffer motor neuronal deficits, but it has never been determined whether this is a general effect, or specific to a given subpopulation. New research by Thomas Gould of the Laboratory for Neuronal Differentiation and Regeneration (Hideki Enomoto; Team Leader) reveals that, somewhat surprisingly, loss of the function of GDNF or its receptors affects only the gamma motor neurons, which innervate intrafusal fibers. The study, published in The Journal of Neuroscience, further highlighted the exquisite specificity of GDNF signaling function by showing that the period in which GDNF is needed for motor neuron survival is limited to the first few days after birth. In starting their work, Gould et al. were confronted by a number of technical challenges in teasing out the details of GDNF function, the thorniest of which is that mice entirely lacking GNDF function die at around the time of birth due to dysfunctions in kidney and gut. “The enormous repertoire of mice in the lab made this sort of thorough analysis possible,” says Gould. “”Our conditional mutant mice permit the deletion of GDNF receptors in specific tissue types such as motor neurons, which avoids the lethal phenotype caused by constitutive deletion and thus facilitates the investigation of the role of GDNF signaling in specific regions or cell types.” The difficulty of visualizing and counting motor neurons presented a second hurdle to the team, which it met by developing a new labeling method which they used first to verify the normal number of lumbar motor neurons in wildtype and GDNF-knockout newborn mice. When they looked at different hindlimb muscles, they found that in most muscles innervation was normal in both wildtype and mutant. Only three muscle types – peroneal, gluteus maximus, and iliopsoas – showed deficits, consistent with previous findings showing a role for GDNF in peroneal axon guidance. Intriguingly, however, in the occasional GDNF ligand-receptor knockouts in which peroneal innervation remains normal, the mice still lacked greater numbers of neurons that could be accounted for by deficits in the other two groups alone. This prompted Gould to look at the possibility that rather than those innervating a specific muscle group, motor neurons projecting to a specific muscle fiber type might be affected by loss of GDNF signal. Indeed, whereas extrafusal skeletomotor neurons (also known as alpha motor neurons) were completely unaffected in knockouts of Ret (a vital component of the GDNF receptor complex), this same mutation caused near-total reduction in the number of fusimotor (or gamma) neurons. Similar fusimotor deficits were seen in knockouts of GDNF and GFRα1 (the other half of the Ret receptor complex). Observation of myelinated axons in motor neuron-specific Ret and GFRα1 conditional knockouts confirmed that this deficit was confined to small-diameter, gamma motor neurons. Finally, the analysis of mice expressing the fluorescent reporter CFP in one or both Ret loci (Ret-CFP knockin mice) revealed that the reductions in gamma motor neuron counts at least partially result from programmed cell death during initial innervation of intrafusal muscle fibers. The team also crossed mice with conditional mutations in either Ret or GFRα1 to those expressing a tamoxifen-inducible Cre to study the requirement for GDNF at different points in fetal and postnatal development, and found that gamma motor neurons depend on the GDNF pathway only during the late developmental period during which cell death occurs.
“In addition to the biology, what we found has some interesting implications for medicine,” says Gould. “In ALS [amyotrophic lateral sclerosis], a fatal degenerative disease characterized by the selective loss of alpha MNs, for example, it had been thought that the loss of alpha motor neurons might be an instance of dysregulation of GDNF signaling. But our findings show that it is the gamma neurons, rather than the alpha neurons, that appear most sensitive to the trophic actions of GDNF, which raises questions about the clinical relevance of this pathway in the pathogenesis of ALS.”
|
|||||
|
|||||
|
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |