| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
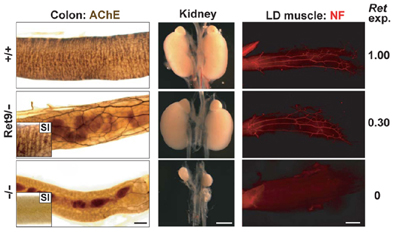
June 5, 2008 – The long tract of the gut is innervated by a network of peripheral neurons that control, among other functions, the peristaltic action that moves food through the lower half of the alimentary canal. In Hirschsprung’s disease, a congenital disorder, neurons fail to develop in the large intestine, which can lead to symptoms ranging in severity from chronic constipation to total blockage and enlargement of the colon, which can be life-threatening. The primary genetic cause of Hirschsprung’s has been linked to mutations in the Ret gene. Recent evidence has also shown that the binding of the protein GDNF (glial cell line–derived neurotrophic factor) to its receptor GFRα1 activates RET, which is necessary for the survival of enteric neurons, leading to the question of precisely how Ret regulates neuronal survival and death in the developing colon, particularly with reference to Hirschsprung’s disease. Toshihiro Uesaka and colleagues in the Laboratory for Neuronal Differentiation and Regeneration (Hideki Enomoto; Team Leader) have now developed a mouse model of Hirschsprung’s in which Ret gene expression levels can be reduced conditionally, allowing them to study the gene’s involvement in an animal model with greater precision than ever before. This work was published in The Journal of Clinical Investigation. Uesaka had previously shown that loss of GFRα1 function at a specific stage late in mouse development caused a near-total loss of innervation in the large intestine. “In the conditional GFRα1 mouse, we found an almost complete loss of the neuronal network in the lower intestine due to apoptotic cell death, a phenotype that closely resembles Hirschsprung’s disease,” says Uesaka. “As this disease is characterized by mutations in the Ret gene, we suspected that apoptosis might be involved there as well.” In mice in which GDNF, GFRα1 or Ret are ablated unconditionally, the enteric nervous system fails to develop beyond the stomach. In the sense that it produces a loss of the enteric neural network, this phenotype could be said to resemble Hirschsprung’s disease, but in fact such extreme cases are rare, and many patients show only a loss of neurons in the colon. Previous reports have also shown that Ret-mutant mice exhibit abnormal kidney and motor neuron development as well, which is not seen in Hirschsprung’s, indicating the need for a more sophisticated experimental model. “By designing a system for conditionally inactivating Ret at a specific developmental stage in the mouse fetus, we were able to confirm that, just as with GFRα1, the loss of Ret function at a specific developmental stage results in a reduction in the innervation of the colon,” comments Uesaka. “We knew that in human Hirschsprung’s patients, there’s a mutation in the Ret gene of a region that regulates its expression level, so we next looked at a series of mutant mice in which Ret expression levels were reduced to various extents.” Mice in which Ret expression was reduced to 50% of the wildtype level were asymptomatic, but in a different mutant in which levels were 30% of wildtype, colonization of the gut by enteric neural crest–derived cells initially appeared to play out as normal, but these cells subsequently disappeared. This phenotype occurred in about half of all the animals, in a gender distribution reminiscent of that in Hirschsprung’s. The phenotype was limited to gut innervation without any of the other defects, such as kidney and motoneuron anomalies, that are seen in unconditional Ret knockouts. And, interestingly, the gender distribution showed a slight male bias, consistent with the tendency in the Hirschsprung’s patient population.
Uesaka next looked into the mechanism by which low levels of Ret expression might bring about such neuronal loss, and found that in the mutants neuronal migration into the colon is delayed. Reductions in Ret induced at later developmental stages also caused a loss of colonic innervation, suggesting that lower Ret levels may both interfere with the survival and maintenance of enteric neurons and trigger the programmed cell death that underlies Hirschsprung’s pathogenesis. “It had previously been thought that Hirschsprung’s disease was caused by a defect in cell migration, not by cell death,” says Uesaka. “But our findings suggest that insufficient supply of a survival signal is at fault. I am hoping to look more closely at the underlying mechanisms in the future to contribute to a better understanding of this disease.”
|
|||||
|
|||||
|
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |