| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
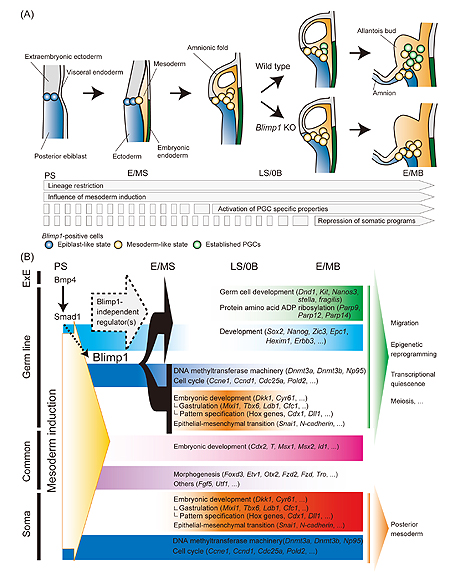
June 20, 2008 – Evolution has devised two ways for the germline to arise during development: through preformation, in which germ cells are determined by maternal factors segregated into a few cells from the very beginning of development, and epigenesis, in which signals delivered by the embryo itself instruct undifferentiated cells to adopt germline fate. Several popular model organisms, including C. elegans and Drosophila, take the preformation route, but most species, including mammals such as human and mouse, feature epigenetic germline development. So the question of how genetic signals induce the first few germ cells is one of fundamental importance and interest. A new genome-wide study of transcriptional dynamics conducted by Kazuki Kurimoto and others in the Laboratory for Mammalian Germ Cell Biology (Mitinori Saitou; Team Leader) now reveals how a complex network orchestrated by the regulatory factor Blimp1 guides the specification of primordial germ cells (PGCs) in the mouse. This work, which brought a sophisticated single-cell microarray and PCR analytical approach to bear on the question of what sets these cells apart from their somatic neighbors early in mammalian development. The article was published in the journal Genes and Development. “The entire mouse germline arises from a small group of about 40 cells quite early in the embryo’s development,” says Kurimoto, “which meant we had to be able to analyze very small samples. The single-cell technology developed in our lab a few years ago was essential to making that possible.” The study, which looked at transcriptional dynamics and gene interactions across the entire genome in both wildtype embryos and those carrying mutations in the definitive PGC marker Blimp1 (also known as Prdm1), represents the first such comprehensive study in any mammalian cell lineage. The team began by comparing gene expression patterns in cells taken from embryos at 6 different stages of development between day E6.25, when the germline is first specified, and 8.25, when the somites begin to appear and germline commitment is complete. One mainstream theory of germline specification has it that Blimp1 acts to repress “somatic” genetic programs, such as those enacted by Hox genes (represented here by Hoxb1). The picture that emerged from this close analysis, however, was a bit more complex: undifferentiated cells (marked by the gene Sox2) that become specified by Blimp1 for germline fate first transiently express Hoxb1 at levels similar to those in the surrounding soma before ultimately repressing this and other somatic genes. Comparison of genome-wide expression patterns in wildtype and Blimp1-mutant embryos also shed light on the dynamics and functions of specification and somatic genes in the germline anlage. Among the highlights of the spectrum of new insights were the findings that nearly all genes relating to the establishment or maintenance of DNA methylation are repressed in PGCs, and that germline specification occurs without a requirement for epithelial-mesenchymal transitions. This comprehensive trove of gene expression profiles of cells undergoing PGC specification now allows a more detailed understanding of what distinguishes the nascent germ lineage from its somatic neighbors at the genome-wide scale. Kurimoto’s analysis of loss-of-Blimp1–function embryos yielded even more intriguing gold, with the revelation that while this gene certainly plays a master role, it apparently does not work alone. While Blimp1 was essential for the repression of almost all of the genes normally downregulated in PGCs, the transcription of many genes that had been presumed to be under the sole control of Blimp1 was unaffected in the mutants, suggesting that other factors must also play a part. “What we saw was that some genes specific to the germline would switch on to some extent even if Blimp1 wasn’t working,” says Kurimoto. “That said, in the mutant, the individual cells still couldn’t coordinate all the many genes required. It seems that Blimp1 functions something like the conductor of an enormous orchestra, making sure that everything plays according to the score.”
|
|||||
|
|||||
|
|
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |