| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
June 23, 2008 – The cells of the embryo can be classed roughly by their structures into epithelial and mesenchymal types; epithelium is characterized by its more rigid borders, clear apical-basal polarity, and well-defined physical junctions with other cells and the extracellular basement membrane, while mesenchymal cells show none of these interactions and have more free-form morphologies. These cell types, however distinct, are not permanent, and switches from epithelium to mesenchyme or vice-versa occur in many developmental contexts, a phenomenon known generally as epithelial-mesenchymal transition (EMT) or mesenchymal-epithelial transition (MET). Given the frequency and importance of such events, they have been widely studied in cultured cells, but due to the technical challenges it presents, the regulation of EMT in vivo has yet to be adequately investigated.
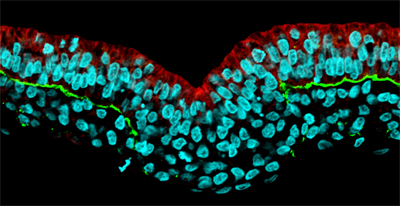
Working with chicken embryos, Yukiko Nakaya and others in the Laboratory for Early Embryogenesis (Guojun Sheng; Team Leader) have now shown that EMT during gastrulation plays out as a serial, stepwise process, beginning with the breakdown of the basement membrane. In an article published in Nature Cell Biology, Nakaya reports that this initial stage in the epithelial-mesenchymal transition is controlled by the downregulation of the factor RhoA, a small Rho GTPase known to regulate cytoskeletal rearrangements. Gastrulation is a process that takes place during early development in which, through a series of tissue movements, the early mammalian embryo transforms itself from an almost featureless cylinder to a rudimentary but recognizable body composed of three germ layers: ectoderm, mesoderm and endoderm. In the chicken, epithelial cells from a structure known as the epiblast ingress to form mesoderm (the germ layer that gives rise to tissues including heart, blood, bone and muscle), requiring them to undergo EMT in which they dissolve their attachments to neighboring cells and the underlying extracellular substrate. “The epithelial-mesenchymal transition involves a series of distinct events that fortunately can be observed clearly and directly in the chicken embryo,” says Nakaya. “What we wanted to do here was look at RhoA in this context, as it was known to play a part in regulating cytoskeletal changes in cultured cells, but was not very well studied in the living embryo.” The team first used electroporation to inject the RhoA gene into the chicken epiblast, and found that its misexpression caused a failure in the normal detachment of the epiblast from the basement membrane, a component of the extracellular matrix, preventing the occurrence of EMT. Looking more closely at the mechanics, Nakaya found that overexpression of RhoA caused a stabilization of basal microtubules, a cytoskeletal component responsible for maintaining cellular structure, which served to mediate the cell-basement membrane interaction. Inhibition of microtubules by a chemical agent caused the opposite effect, the breakdown of the basement membrane expanded beyond its usual region, a phenotype that could be induced by loss of RhoA function as well. The findings from this study point to a scenario in which the interaction between epithelial cells and the basement membrane is mediated by the stabilization of microtubules by RhoA. The down-regulation of RhoA in the epithelial epiblast allows for cells anchored by this cell-substrate interaction to break free and adopt the mesenchymal state, a critical first step in the EMT process. “Much of the work that has been done in EMT to date has focused on experiments done in vitro, so there have been a lot of calls to look at it in vivo as well,” says Nakaya. “What we’ve been able to do in this study is identify an important new player in an EMT event taking place in a living embryo. And because EMT is not limited to embryonic development, but is very important in the invasion and metastasis of epithelial cancers as well, I hope more people will come to recognize the value of the early chicken embryo as a model for studying how it works.”
|
|||||
|
|||||
|
|
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |