| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
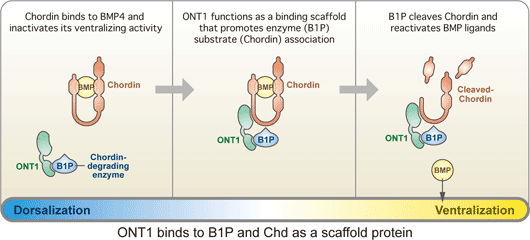
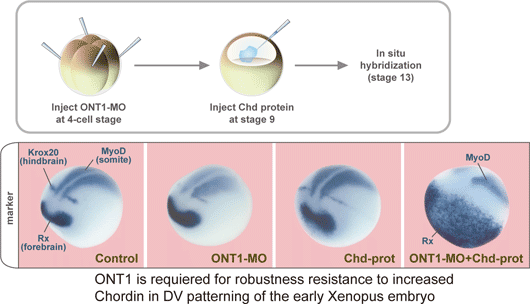
September 15, 2008 – One of the key questions in development is how tissues know when to stop growing. Early experiments on the head organizer by Hans Spemann and colleagues famously showed that this signaling region can prompt the growth of a second dorsal axis when transplanted into the ventral region of an amphibian embryo. Interestingly, the features, such as heads, that develop from these explants maintain similar sizes to those found in wildtype embryos, suggesting that dorsal-ventral developmental routines are robust against massive perturbations in their regulatory environment. But while the interplay of factors that assigns back and belly fates is well characterized, it has never clearly been shown how this robustness in the face of change is maintained. A study by Hidehiko Inomata and colleagues in the Laboratory for Organogenesis and Neurogenesis (Yoshiki Sasai; Group Director) has identified a new factor responsible for stabilizing the dorsal-ventral axial pattern in Xenopus laevis, a popular system for the study of signaling in early development. This work, published in the journal Cell, shows that a secreted scaffold protein, ONT1, the neural inducer Chordin (a BMP antagonist) into contact with a degradatory enzyme, allowing it to fine-tune BMP signaling in the axial tissue. The study emerged in part from a theoretical conundrum surrounding the network of dorsal-ventral regulatory factors: the mutually inhibitory relationship between dorsalizing (such as Chordin) and ventralizing proteins (BMP4) appeared to be vulnerable to instabilities capable of triggering catastrophic changes in the patterning of the embryo. The observation that rarely occurs under either natural or experimental conditions opened up the possibility that some unknown factor might be at work restoring the axial balance when things begin to go astray. One such factor, ADMP, was shown in a study by a different lab to work in this manner, but its loss-of-function phenotype suggested that it alone could not account for the sturdy robustness of this system. Inomata’s work began with the identification of the protein ONT1 in chicken by others in the Sasai lab. A member of the olfactomedin family and related to other neurodevelopmental regulatory proteins such as Noelin and Tiarin, ONT1 was interesting also for the expression of its homolog in the dorsal side of the embryo, from which the nervous system arises in frog. Using morpholinos to study its knockdown phenotype, the group found that loss of ONT1 function resulted in dorsalization as evidenced both by morphology and gene expression. Wanting to know more about how it might relate to other components of the BMP regulatory network that controls dorsal-ventral patterning, they used tagged ONT1 in immunoprecipitation assays against a range of BMPs, their receptors and modulators, as well as other candidates such as Wnt and Nodal. Strong affinity was shown for the BMP antagonist Chordin, but also intriguingly with a group of proteinases (collectively referred to as B1TP proteins) known to degrade Chordin. Analysis of deletion mutations showed that the interactions with Chordin and the B1TP enzymes depend on different ONT1 domains. This dual role of ONT1 I yoking Chordin to enzymes brought up the possibility that this protein works to titrate Chordin levels in dorsal-ventral patterning. Despite the apparently primary role of Chordin as a BMP antagonist, this inhibitory system is quite robust in response to changes in Chordin availability. Knowing that even a significant gain of Chordin function usually has only moderate dorsalization, Inomata tested whether the combination of Chordin overexpression and ONT1 deletion would disrupt the system’s stability, and found that it did result in a hyperdorsalized phenotype as measured by gene expression and morphology.
In vivo tests of ONT1 at different concentrations showed that its role as a scaffold linking Chordin and B1TP enzymes is dose-dependent; normal levels of ONT1 reduce Chordin by promoting its degradation but, seemingly paradoxically, its overexpression actually leaves Chordin protected by binding to individual proteins and preventing the enzymes from coming into contact with and digesting their target. This scaffold model was further supported by tests showing that the protection of Chordin on ONT1 overexpression is lost when a B1TP enzyme is also overexpressed, as well as (at a molecular level) immunoprecipitation assays that revealed increased Chodrin-B1TP association in the presence of ONT1.
Interestingly, the group found that ONT1 is negatively regulated by ventralizing BMP signals, just as is Chordin. Given that its mode of action as a facilitator of Chordin degradation differs from ADMP, a previously reported pro-BMP (or to be more accurate, anti-anti-BMP) factor also shown to have only a moderate loss-of-function phenotype, they performed a double knockdown to see if the two work in concert. The ONT1-ADMP morphant indeed showed much stronger dorsalization, suggesting the factors play complementary roles, a kind of double safeguard that underlies the dorsal-ventral system’s robustness. “Past studies have developed a pretty clear picture of deterministic events in development,” says Inomata, “but we have a poorer of understanding of how developmental programs recover when confronted with potentially catastrophic perturbations. This work shows that there is a kind of emergency manual for dealing with crisis situations, and maintaining this system’s robustness.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |