| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
October 15, 2008 – The past decade has seen dramatic progress in our understanding of the molecular factors that direct the early development of the ear. It is known that, in both chicken and mouse, FGF signaling from the mesoderm initiates the induction of the inner ear, and that Wnt signals from the overlying neural plate also play a role. But the order of events in this stepwise process, and the specific functions of these two signaling pathways in inner ear induction have proven elusive. New work by Sabine Freter and colleagues in the Laboratory for Sensory Organogenesis (Raj Ladher; Team Leader) now provides a higher resolution view into this problem. In an article published in Development, the team reveals how FGF and Wnt work in setting up and then progressively restricting the fate of the embryonic region that ultimately gives rise to the inner ear.
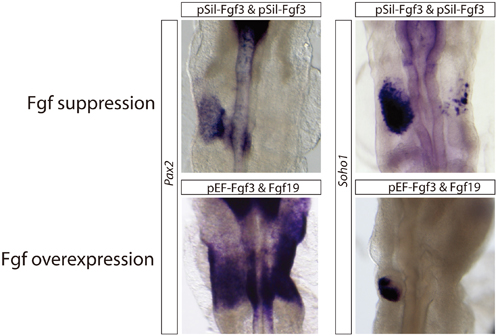
Findings from previous studies, in which it was found that explants from the same pre-otic region isolated from embryos at closely spaced developmental stages (defined by number of somites) showed differing expression of certain genes. To see if this would hold true for genes used in inner ear induction, Freter removed presumptive explants at the 5-somite stage and tested for the otic marker Soho1, finding only minimal expression. When she took explants from the same region at the 7~8 somite stage, however, they showed high levels of Soho1 transcription. Returning to previous findings from the Ladher team which showed that FGF signaling is needed to trigger the first steps toward otic differentiation, but that FGF expression subsequently fades in the region, Freter decided to test whether the downregulation of FGF was merely incidental, or in fact a prerequisite for subsequent stages in the process. Using a genetic trick to leave FGF permanently switched on, and found that, while it worked to expand the field of tissue from which the inner ear ultimately should emerge (which they call the otic-epibranchial progenitor domain, or OEPD), genes needed to induce differentiation into the inner ear proper failed to switch on. This came as something of a surprise, as the link between FGF expression and otic development is well established. Indeed, when the team tried suppressing FGF signaling, they found again that the development of the inner ear failed. (Interestingly, the expression of non-otic genes was unaffected by constitutive FGF.) In chick, a second signaling pathway known as Wnt has also been implicated in otic development. Using a mutant downstream of Wnt that results in the pathway being constantly activated, Freter looked for effects in the otic and non-otic areas of the OEPD. In a near mirror image of the case for FGF in the second stage of otic development, she found that Wnt was required for otic development, but not so for the epibranchial regions of the OEPD. This sets up a delicate balance between the programs needed to drive otic and non-otic differentiation from a common progenitor region that is induced by FGF. Whereas otic differentiation relies on the combination of Wnt signaling and downregulation of FGF, epibranchial differentiation requires FGF to be maintained and Wnt shut off. “This idea that a factor required for one stage in a multistep developmental process can actually have an inhibitory effect on subsequent differentiation of certain lineages was surprising to us at first, but now seems to make a great deal of sense,” says Freter. “It will be interesting to see if this is a common feature in the genetic control of other developmental routines.”
|
||||||
|
||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |