| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
October 25, 2008 – Gene expression is controlled not only by regulatory elements that bind to the DNA, but by the way the DNA itself is packed away in chromosomes. These structures are composed of a combination of DNA and proteins known as chromatin, in which the genetic material is wrapped up into tight coils. In its “open” conformation (euchromatin), genes tend to be more transcriptionally active than in heterochromatin, where gene silencing is common. This transcriptional silencing in heterochromatic regions is controlled by various factors, including members of the HP1 protein family, but whether and how these proteins interact to achieve this function remains unknown. Mahito Sadaie and colleagues in the Laboratory for Chromatin Dynamics (Jun-ichi Nakayama; Team Leader) have now shown how, in fission yeast, the two HP1 proteins Swi6 and Chp2 play distinct, but cooperative, roles in heterochromatin assembly. In an article published in Molecular and Cellular Biology, the team revealed that while Swi6 contributes to the repressive structure through the ability of these molecules to self-associate, Chp2 functions by recruiting other chromatin-modulating factors.
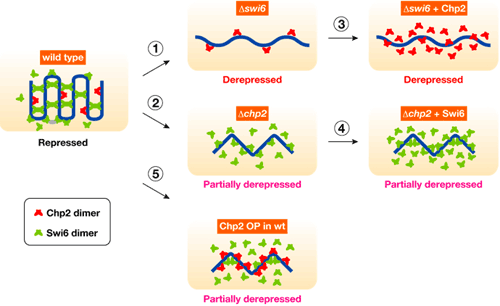
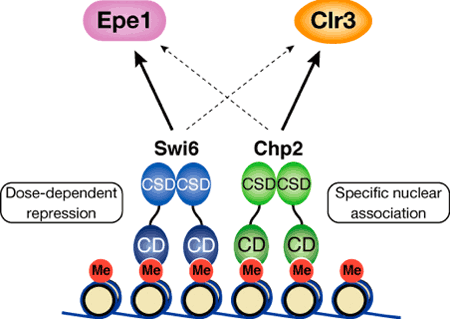
Both Swi6 and Chp2 had previously been linked to heterochromatin assembly, but it was not clear whether their roles were discrete, or simply redundant. Taking advantage of yeast genetics, Sadaie first tested whether there was functional overlap by complementary expressing Chp2 in a swi6-negative mutant strain, and Swi6 in a chp2 mutant. In neither case was the transcriptional silencing defect rescued, suggesting that both were required in this mechanism, but each in a different way. One clear difference between the two is their levels of expression – Western blot analyses showed that the Chp2 protein was only about 1/80 as abundant as Swi6, indicating differential regulation. The team found that this asymmetric balance was functionally important, for when they overexpressed Chp2 in cells expressing normal levels of Swi6 it disrupted heterochromatin assembly. Seeking possible molecular interactions, Sadaie performed pull-down assays and found that while Swi6 proteins bind with other proteins of the same, Chp2 showed no such self-association. The team turned next to studying how Swi6 and Chp2 are involved in the recruitment of other chromatin-regulating factors to heterochromatic regions. Swi6 was known from previous reports to influence the binding of a pair of factors, Epe1 and Clr3, to heterochromatin; Sadaie et al. showed that Chp2 also physically associates with Epe1, and that it has a higher affinity for Clr3 than does Swi6, meaning that it is likely to play the dominant role in recruiting this protein to heterochromatic regions. The case for this interaction was further strengthened by experiments showing that the overexpression of Clr3 rescued the silencing defect in Chp2 mutants.
The picture that emerges from these findings is that, in fission yeast at least, heterochromatin organization relies on the proper balance between two members of the same protein family, each of which plays a distinct role. “It may not be possible to extrapolate these results directly to other species,” says Nakayama, “but the mechanisms we have found for the dynamic regulation of heterochromatin and gene silencing in fission yeast may enable a deeper understanding of how similar functions are achieved in mammals,” |
|||||||||
|
|||||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |