| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
December 11, 2008 – Controlling organ growth, or cell proliferation, is one of the keys to ensuring that the body’s parts grow to the appropriate size and no bigger. When this control is dysregulated by, for example, aberrances in the cell cycle it can lead to hypertrophy or cancer. In cultured cells, a phenomenon known as cell contact inhibition of proliferation is one means by which groups of cells self-regulate their growth; at higher densities proliferation is slowed as cells come into closer contact with their neighbors. Cancerous cells, however, lose this inhibitory control and growth runs rampant as a consequence. Contact inhibition is thought to be a crucial mechanism in development as well, but the details of how it works on the molecular level have remained obscure.
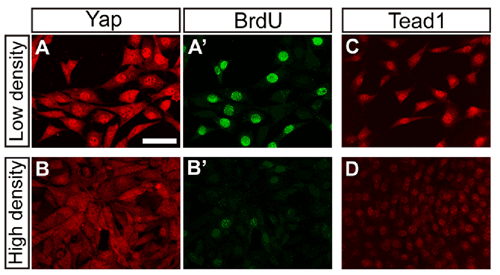
Now, a study by Mitsunori Ota of the Laboratory for Embryonic Induction (Hiroshi Sasaki; Team Leader) has yielded new insights into the molecular mechanisms that underlie cell contact inhibition in mouse. Reporting in the journal Development, the team showed that a transcription factor, Tead and its co-activator protein Yap1, exert control over proliferation by mediating Hippo signaling, a pathway associated with tumor suppression in Drosophila. The work began almost by accident, when Ota was investigating expression of Tead and Yap1, which have been previously identified in Sasaki’s laboratory as key regulators for development of the node and notochord inductive signaling centers in the mouse embryo. The turning point came when he tested Yap1 in cultured cells, and found a link between cell density and Yap1 localization. In low-density culture conditions in which cells divide rapidly, Ota found that Yap1 remained in the nucleus, but in higher-density conditions where growth is inhibited, the protein moved into the cytoplasm. “that was the first time I suspected there might be a link between Tead, Yap1 and cell contact inhibition of proliferation,” says Ota. Tests of Tead activity in cells cultured at different densities confirmed those suspicions. Knowing of the role of Hippo signaling in cell proliferation, Ota next examined whether it might be involved in the regulation of Tead. Constitutive expression of Hippo pathway components caused the downregulation of both nuclear Yap1 and Tead activity, indicating that cell contact modulates the activity of Tead proteins through Hippo pathway. The converse experiment, in which Tead was activated by misexpression of Yap1 or activated forms of the Tead protein, resulted in upregulation of growth, suppression of apoptosis, and promotion of tumorigenesis, supporting the case for roles for Yap1 and Tead in controlling cell proliferation. This prompted them to look for the gene targets of these factors, using microarrays to analyze expression profiles. Their tests showed considerable overlap in the genes controlled by the two factors, suggesting that both work on similar targets to regulate growth. But on looking more closely in vivo, a more nuanced picture emerged. Using embryos with a homologous deletion of the two major Tead genes, or of Yap1, they found that the expression of only a small fraction of the genes induced by Tead in their cultured cells was affected in the knockout embryos, suggesting that these factors regulate different targets in a cell type-specific manner. Protein distribution patterns showed diversity as well. While Tead1 was expressed in all tissues in embryos between days 8.5 and 10.5, it was particularly strong in heart muscle. Similarly, Yap1 was widely expressed, with the highest levels in the node and notochord, as well as in myocardium, where it co-localized with Tead. These findings point to cell type-dependent differences in the Hippo pathway, which regulates contact inhibition of proliferation by its effects on Yap1 and, consequently, Tead. “We knew from studies in fly that Hippo was linked to the control of organ size,” says Sasaki, “what we’ve done here is show not only that it plays a role in cell contact inhibition of proliferation in mouse, but that the pathway involves Tead proteins. We have been studying other roles for Tead as well, so we’ll be interested to see how the Hippo pathway and cell contact information as well fit into the greater picture of mammalian embryogenesis.” |
||||||
|
||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |