| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
December 26, 2008 – Studies of how the body is put together tend to focus on cells as the building blocks of tissues and organs, but the extracellular matrix, the collective name for various meshworks of proteins and complex carbohydrates that play a vital role in holding the body’s diverse cells together. Basement membranes are one such extracellular matrix, comprising layers of collagen and other fibrous proteins and proteoglycans, which serve the important functions of anchoring epithelial tissue and providing a barrier between the body’s compartments. The migration of distal tip cells (DTCs) during gonadogenesis in C. elegans has been used as a relatively simple system for studying the part played by basement membranes in organ formation. But though it has been shown that an ADAMTS family protease known as MIG-17 is required for controlling the direction of the distal tip cells as they followed their U-shaped path through the larval body, possibly by remodeling the basement membrane, but the picture downstream of MIG-17 remained unclear.
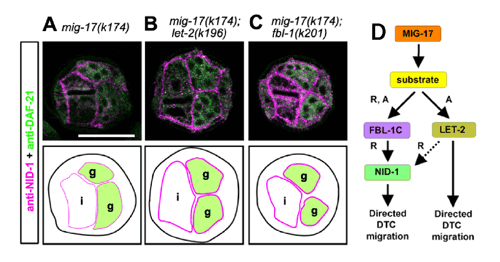
A new study by Yukihiko Kubota, Kiyotaka Ohkura and colleagues in the Laboratory for Cell Migration (Kiyoji Nishiwaki; Team Leader) has now revealed a new network of proteins which, in mutant form suppressed the defects induced by mutation of MIG-17. Interestingly, these suppressor mutations (in the genes let-2 and fbl-1) appear to work by distinct mechanisms. The study began with a screen for genetic defects affecting gonadal migration, using mig-17 mutant worms as a starting point. Previous work by the Nishiwaki lab had identified one such suppressor mutant, in which a mutant form of the fibulin-like protein encoded by fbl-1 suppresses MIG-17 defects in a dominant gain-of-function manner. The mutagenesis screened turned up two new mutations in the gene let-2 (which encodes a C. elegans version of collagen IV), both of which bypass the mig-17 migration defect. Although neither let-2 nor fbl-1 mutants alone show significant migration phenotypes, when Kubota and Ohkura introduced mutations into both genes, they found severe gonadal defects, suggesting a genetic interaction. They next looked at another basement membrane protein, nidogen, which is known to bind to type IV collagen, in order to determine whether it played a part in the equation. But although nidogen and MIG-17 appeared to be at least partially redundant, the combination of mutant nid-1 with either of let-2 collagen mutants had no effect on their suppression of mig-17-induced migration defects. The loss of nid-1 had a strong effect, however, on the ability of the fbl-1 mutation to suppress the mig-17 phenotype, indicating that the two suppressor mutations of let-2 and fbl-1 operate respectively by nidogen-independent and -dependent mechanisms. As nidogen normally binds to collagen in the basement membrane, the team checked whether this was affected in the various migration-related mutations, alone or in combination. Using immunohistochemistry, they found that mutations in mig-17 and let-2 had weakened on nidogen localization, while loss of fbl-1 function caused a more dramatic deficit. Intriguingly, the introduction of either of the suppressor mutations in a mig-17 mutant background amplified the accumulation of NID-1. Overexpression of NID-1 in mig-17 mutant worms significantly rescued the gonadal defect, suggesting that its accumulation in the basement membrane plays an important role in mig-17-dependent control DTC migration. The picture that emerges is complex, and still incomplete. Although the details of the relationship between FBL-1 and NID-1, and the precise role for LET-2 in the regulation of migration along the basement membrane remain for further study, the findings of Kubota, Ohkura, et al. point to a much more elaborate role for the extracellular matrix in organogenesis than previously suspected. “We know of a number of genetic diseases involving ADAMTS proteases associated with changes in the extracellular matrix, but how these proteins affect the ECM is largely unknown,” says Nishiwaki. “We have now found that in C. elegans, MIG-17/ADAMTS recruits factors to the basement membrane in a determined order, giving us one of the first insights into the molecular control of organogenesis by this system.” |
||||||
|
||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |