| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
May 18, 2009 – Segregating the germ cells used in reproduction from the rest of the body’s (somatic) cells is a mission critical task for the embryo, for more than any organ system, the germline is the body’s raison d’ętre. In many species, the initial commitment of the germ lineage cells takes place early in development, and sets of them off on a journey through somatic regions before they reach their final home in the gonads. This is true for mammals as well; in the mouse, the roots of the germline have been traced back to as early as the sixth day of development. But what triggers the expression of the first germline-specific genes has been a mystery.
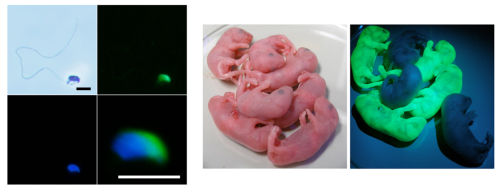
A new study by Yasuhide Ohinata and colleagues in the Laboratory for Germ Cell Biology (Mitinori Saitou; Team Leader) now reveals signals even further upstream in the germ cell specification cascade, originating from the extraembryonic ectoderm. Reported in Cell, the newly unearthed mechanism, which involves both the Bmp and Wnt pathways, was further shown to drive the high-efficiency differentiation of this tissue to the germ lineage in vitro, yielding viable germ cells (sperm) capable of fertilization following transplantation into testes. The importance of Bmp signaling, including the Smad proteins downstream, from the extraembryonic ectoderm had been pointed at by earlier work from other labs, while Saitou’s lab had previously identified Blimp1, Prdm14 and stella as the earliest genetic factors in germline specification. Taking that as their starting point, Ohinata and colleagues asked how these two knowns might be connected. In the embryo, the germline specifying factors Blimp1 and Prdm14 are expressed in only a limited region of the epiblast proximal to the extraembryonic ectoderm, while Bmp4 and Bmp8 are uniformly expressed throughout that tissue. Loss-of-function studies revealed that germ cell specification is the result of an interaction between two signaling axes – one related to proximity to the extraembryonic ectoderm (involving the Bmps) and a second that represented posterior epiblast properties, which could be inhibited by an antagonistic signal from the anterior visceral endoderm (via a pathway mediated by Smad2 and FoxH1). They next tried culturing these embryonic regions to see if their insights into the specification mechanism could be replicated in vitro. Using Day 6 epiblast and extraembryonic ectoderm, they showed that the addition of Bmp4 to the culture medium was sufficient to induce the expression of the germline-specifying factor Blimp1 throughout the entire explanted region. Further studies using mutants for the Wnt factor Wnt3, which is involved in early embryonic patterning, showed that Bmp4’s instructive function is Wnt-dependent. Interestingly, a second Bmp factor, Bmp8b, which is known to be important for primordial germ cell, had no direct effect on germ cells. Using a mouse model in which the Bmp8b gene had been knocked out, and further in vitro studies, Ohinata et al. determined that the role of this protein is to inhibit development of the anterior visceral endoderm, which itself has an inhibitory effect on Bmp4. With this solider understanding of germ cell differentiation, Ohinata used the culture system the lab had developed to steer epiblasts to adopt this fate. The induced cells showed expression of Blimp1 and other genetic and epigenetic hallmarks of germ cells. The real test of their character, though, came with their injection into the testes of sterile mice, which revealed that they were fully capable of further differentiation into viable sperm. “In this study, we looked at the epiblast, which is composed of pluripotent cells that give rise to the embryo, so in that sense what we learned may also be applicable to other pluripotent cells, such as embryonic or induced pluripotent stem cells,” says Saitou. “Although some ethical questions remain, it may be possible to apply this technique for germ cell differentiation in the future, which may have applications in the treatment of infertility in addition to its fundamental scientific interest.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |