| Opposing roles for Wnt receptors in the olfactory system |
 |
 |
June 10, 2009 – Olfaction is one of the primary sensory modes by which many species, including insects, survey their environments. The olfactory system is therefore highly specialized and exquisitely sensitive to a very broad spectrum of odorant signals. The fruit fly Drosophila melanogaster, for example, has around 1300 olfactory neurons each carrying one of around 60 olfactory receptors. Adding to the complexity, these neurons bundle together into 50 distinct glomeruli, which can be found arrayed in a consistent pattern in the brain of any given fly. The control mechanisms responsible for ensuring this olfactory patterning are known to involve the signaling factor Wnt5, but otherwise remain largely a mystery.
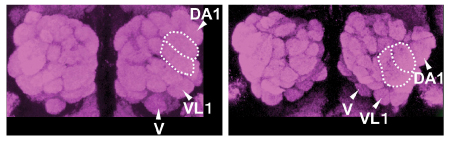 |
Multiple glomeruli in the Wnt5 mutant brain (right) are displaced in a clockwise fashion
compared to their positions in wildtype (left) |
New work by Makao Sakurai and colleagues in the Laboratory of Neural Network Development (Chihiro Hama; Team Leader – now a CDB Senior Investigator) reveals that a pair of receptors – Drl and Drl-2 –are expressed in different olfactory cell types and play opposing roles in Wnt signaling. These findings, published in The Journal of Neuroscience, shed new light on the cooperative mechanisms at work in establishing the olfactory circuitry.
The study began by looking at Wnt5 mutants, which Sakurai found resulted in a phenotype in which peripheral glomeruli in the antennal lobes rotated clockwise of their wildtype positions. Knowing the role of Drl as a Wnt5 receptor, he next checked the drl mutant phenotype, but found it to be substantially different from that of Wnt5, suggesting that other receptors might also be involved. To test this possibility, the team engineered flies to lack function of both drl and a related gene drl-2, and found that the double mutant phenotype much more closely resembled that of the Wnt5 mutant, which suggests that both Drl and Drl-2 function as Wnt5 receptors.
One reason for the differences in the Wnt5 and drl phenotypes is traceable to Drl’s antagonistic effect on Wnt5 signaling. The Drl protein is expressed in only a subset of second-order olfactory neurons and glial cells, and it is in the latter that the repressive function manifests. Drl-2 in contrast is normally expressed in the axons of olfactory receptor neurons. But when the Hama team misexpressed Drl-2 in the glial cells of drl mutants, they found it was capable of compensating for the loss of drl function, which antagonizes Wnt5 signaling. This led to the somewhat enigmatic conclusion that these two structurally related molecules play contrasting roles with respect to Wnt5, and that further, the choice of role is determined by the cells in which they are expressed.
|