| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
July 8, 2009 Mouse embryonic stem (ES) cells, distinct from their human counterparts, rely on a cytokine named leukemia inhibitory factor (LIF) to keep them in their pluripotent, self-renewing state; deprived of LIF, they begin to differentiate. A number of?transcription factors, namely Oct3/4, Sox2 and Nanog, form the core circuitry of pluripotency and serve as the molecular hallmarks of undifferentiated ES cells, but how LIF integrates with this triad has remained something of a mystery to ES cell biologists. A better understanding of this mechanism could yield important insights into how embryonic cells maintain their pluripotency in the face of internal dynamics and environmental change. New findings from the Laboratory of Pluripotent Cell Studies (Hitoshi Niwa; Team Leader) show that LIF sits at the head of a pair of pathways that interact with separate elements in the pluripotency genetic core. These parallel circuits may help to stabilize the transcription factor network that sustains stem cells in culture. This work is published in the journal Nature.
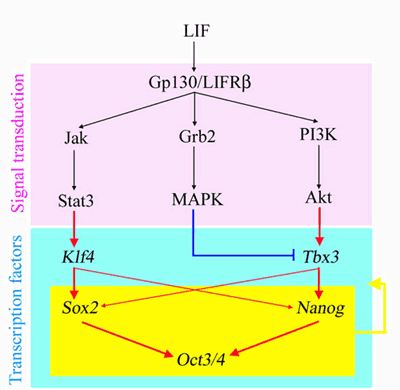
It has been known for some time that a complex, interlocking network of transcriptional pathways is involved in ES cell maintenance, but not all of the pathway components have been identified. LIF is essential for keeping mouse ES cells in an undifferentiated state, but previous work has shown that this requirement can be bypassed by the overexpression of Nanog or the activation of Stat3, a critical factor in a signaling cascade known as the Jak-Stat3 pathway. Other studies involving the analysis of possible targets of the ES cell core circuitry revealed that the transcription factor-encoding genes Klf4 and Tbx3 are also able to compensate for the absence of LIF. Niwa began by trying to piece together the relationships between these multiple factors in maintaining pluripotency. He first looked at the expression levels of pluripotency circuitry elements in mouse ES cells in which the absence of LIF was compensated for by transgenic Nanog, Klf4, or Tbx3. In all three lines, Oct3/4 remained stable, but interestingly in the Nanog and Tbx3 lines, Klf4 expression dropped to levels similar to that of wildtype ES cells cultured without LIF, which was coupled with reduced expression of Sox2. This jibed well with a previous suggestion that Sox2 and Nanog work independently to activate Oct3/4. Staining of ES cells with antibodies to Tbx3, Klf4 and Nanog revealed that even as Oct3/4 levels remained homogeneous, the levels of these three factors vary from cell to cell in an apparently uncoordinated fashion. Somehow these fluctuating expression levels must work together to produce a single, uniform output. Returning to the LIF linkage, Niwa next tried reintroducing LIF to the transgenic ES cells, and found that while neither Tbx3 nor Nanog responded, Klf4 levels changed after LIF was added to the medium. Previous studies of LIF have shown it to work via three separate signaling routes, known as the Jak-Stat3, PI(3)K-Akt, and MAP kinase pathways; in ES cells, LIF is the only factor in control of the Jak-Stat3 pathway, while the other two are multiply regulated. Wanting to identify the roles of each pathway in ES cell maintenance, Niwa used specific inhibitors and activators of each of the downstream pathways before stimulating the cells with LIF. The team found that while Klf4 is downregulated by the Jak-Stat3 blockade, Tbx3 and Nanog were not. In contrast, when the PI(3)K-Akt was activated, Tbx3 and Nanog remained switched on, while Klf4 was unaffected. MAP kinase appeared to work as a negative control on Tbx3; when the MAPK pathway was inhibited, Tbx3 levels rose. The larger picture that arises from this work is one of parallel LIF-activated pathways, which engage in crosstalk to ensure that Oct3/4 remains unperturbed in ES cells. In this study we showed that the pluripotency-associated transcription factors have a hierarchical network structure defined by pathways that integrate external signals," says Niwa. In this scheme, Klf4 and Tbx3 work as signal transmitters while Oct3/4 sits at the core, and Sox2 and Nanog are intermediates. This new model may help to improve our understanding of how pluripotency is robustly maintained under various environmental conditions." |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |