| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
September 2, 2009 – Although the circadian clock is exquisitely tuned to changes in light intensity and many chemical compounds, it is robust in the face of temperature variations. Although this temperature-insensitivity is seen in a wide range of taxa from bacteria to mammals, its fundamental basis has eluded description. One possibility is that the clock mechanism includes at least one biochemical component that is unaffected by temperature fluctuations, but at first blush this seems counter-intuitive, given the highly temperature-sensitive nature of most such reactions. Now, a collaboration of researchers including Yasushi Isojima from the Advanced Computational Sciences Department, RIKEN, Masato Nakajima, Hideki Ukai and others from the Laboratory for Systems Biology (Hiroki R. Ueda; Team Leader), and Joseph S Takahashi’s group from Northwestern University, which was formed to elucidate underlying mechanisms of the circadian clocks by the chemical-biological approach, has identified an essential regulatory component that shows the selective environmental sensitivity that would be required to explain the flexible-yet-robust workings of the biological clock.
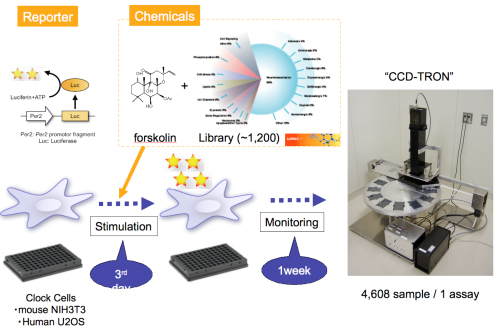
The study began with a screen of more than 1,200 pharmacological compounds to test their effect on clock periodicity, which yielded 10 that had the strongest ability to lengthen clock period in mouse and human cells. The majority of these had never been identified as affecting the length of the circadian period, but two were known to inhibit a family of protein kinases known as CKI (casein kinase I). Taking this lead, the team knocked down various proposed target genes of the 10 compounds to check for possible roles in clock regulation, and found that two – CKIε and CKIδ – had the greatest period-lengthening effects. Going back to their compounds of interest, they found that 9 of the 10 inhibited the catalytic domain of CKIε/δ. CKIε is known to regulate the degradation of an important clock factor known as PER2 (which CKIδ is thought to regulate as well), so Isojima, Nakajima and Ukai next checked whether the two test compounds would inhibit this as well. They found that compounds that putatively inhibit CKI had a significant positive effect on PER2 stability and slowed its degradation, suggesting that these compounds indeed operate via their effects on CKIε/δ. Using the same two compounds, they next tested the extent to which they could lengthen the normal circadian period, and found that both increased the duration of a single cycle by more than double. Using cells expressing a construct of the circadian gene Per2 fused with the gene encoding luciferase to assist in the visualization of its expression, they found that its cyclical expression reflected the changes of PER2 stability seen on administration of the potent compounds, and importantly, that the rate of degradation of the PER2 protein was temperature-insensitive. To confirm whether the underlying CKIε/δ kinase activity is also robust in the face of temperature changes, the collaborators created a peptide substrate derived from the PER2 binding domain thought to be phosphorylated by CKIs, and tested it against the catalytic domain of CKIε at temperatures between 25 and 35 degrees Celsius. Kinase activity remained stable against changes across this range. Interestingly, this temperature-insensitivity was reduced in an autophosphorylating version of CKIε, and when they tested the activity of the kinase with commercially available and general substrates. As a final confirmatory step, they used the Per2-luciferase construct to compare degradation rates in clock cells to those of luciferase alone (which shows temperature-sensitive degradation). As expected, the Per2 constructs significantly outlasted luciferase alone against a range of temperatures, and this effect was intensified by enhancing the reaction by Per2 and CKI by forced expression of the CKIε catalytic domain. These results clearly point to casein kinase I ε/δ factors as the enzymatic drivers behind temperature compensation in the circadian clock. This new knowledge of the basis for period-determination and temperature-insensitivity may additionally prove to be of value in the development of circadian regulating drugs. |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |