| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
March 25, 2010 – DNA famously plays a central role in determining the body’s form and function, typically through the transcription of genes, and the translation of these transcripts to proteins. Controlling this expression of genes, so that they are only “switched on” at the right times and in the right places, makes reliable and precise regulatory mechanisms a necessity. The results of such regulatory activities result in the establishment and maintenance of cells with specific functional identities, or fates. But how is this spatiotemporal transcriptional control achieved?
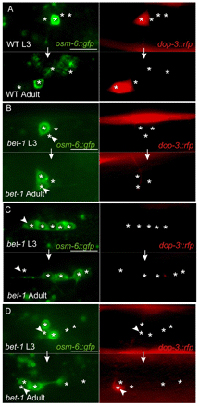
In the somatic germ and other neural lineages as well, loss of BET-1 resulted in the ectopic expression of cell type-specific markers and more than usual numbers of specific type of cells. In at least one case, they traced this effect to the regulation of the expression of a cell-fate determinant by BET-1, a general mechanism that they suspect may be common to other lineages as well. Armed with evidence of the ability of bet-1 mutation to cause cell fate transformations in the somatic germ and neural lineages, the Sawa team suspected that this might also be true in other cell populations. To test this possibility, they observed the expression of marker genes over time in the hopes of gleaning insights into the effects of loss of bet-1 function on cell fate stability. Again, they found that differentiated cells switch between fates in mutants, but not in wildtype. Further tests using heath-shock treatment to conditionally rescue the phenotype suggested that BET-1 establishes and maintains stable cell fates. Pursuing the role of histone acetylation in cell fate establishment and maintenance even further, Shibata next looked at a family of acetyltransferases known as MYST HATs, and found that blocking their function produced phenotypes closely resembling those of the bet-1 mutants. Distribution analyses showed that the localization of bet-1 within the nucleus depends on MYST factors, highlighting a possible functional relationship between the two. These findings shed new light into roles for epigenetic processes in establishing and maintaining cellular identity, possibly through the regulation of the expression of cell fate determinant genes. As analogs of BET-1 and MYST proteins have been implicated in expression of developmental genes in other organisms, such as the fruit fly Drosophila, these factors may have a more generally conserved function in cell fate maintenance and the prevention of wayward differentiation.
|
||||||
|
||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |