| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
March 27, 2010 – When cells divide, they often produce daughters with different identities, sometimes referred to as “fates.” This asymmetric division is an intricate process involving the sorting of intracellular components to different sides of the mitotic cell so as to ensure that they are allocated differentially to its progeny. A growing body of work has revealed that such polarization relies on signals both from within and outside of the cell, and typically involves mechanical action by elements of the mitotic machinery, such as centrosomes. The roundworm C. elegans has served as one of the most powerful systems for studying these phenomena, shedding light on the mechanisms that underlie asymmetric cell division.
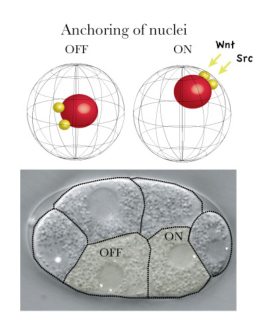
New research by Kenji Sugioka in the Laboratory for Cell Fate Decision (Hitoshi Sawa; Team Leader) examines how the positioning of daughter cell nuclei, a critical event in asymmetric division, is regulated by signaling proteins. Published in the journal Genes to Cells, this work reveals roles for the Wnt and Src signaling pathways in ensuring that cells in the early embryo split up their bequests appropriately. Very early in development, when the roundworm embryo is only four cells, many fundamentally important decisions are being made that will determine whether the embryo develops in the normal fashion. One of these four cells, known as the EMS cell, is the source of the lineages that give rise to tissues such as muscle, gut, and the nervous system. Given its importance, its asymmetric division has been intensively studied at the structural and molecular levels, and it is known that this activity is directed by cues from its neighboring P2 cell in the form of MOM-2 (Wnt) and other signals. Sugioka used 4D live imaging to watch as embryonic cells divided, and made an intriguing observation. First, the centrosomes of the anterior and posterior daughters of the EMS cell behaved differently, with the posterior centrosomes generally moving to anchor, along with the nucleus, to the posterior cortex, while their anterior counterparts rarely did so. Looking at other asymmetrically dividing cells, he found that this posterior nuclear anchoring was widely conserved. Moving back to the EMS cell, Sugioka began to search for a mechanism. Previous work had shown that the EMS cell receives signals from its posterior neighbor, the P2 cell. These signals are mediated by at least two distinct pathways – MOM-2/Wnt through the Wnt/ß-catenin pathway, and MES-1 through the tyrosine kinase SRC-1. To determine whether these signals play a role in nuclear anchoring, Sugioka looked for abnormal nuclear localization in posterior daughters of mutant or RNAi-treated cells, and found that loss of Wnt or SRC function was associated with anchoring defects. But these factors are also involved in the orientation of mitotic spindles, meaning that the effect on nuclear anchoring could be secondary. To rule this out, he quantitatively monitored spindle orientation in three dimensions, and found that even when these structures were oriented within the normal range in MOM-2 or SRC-1 RNAi embryos, nuclear cortical anchoring remained abnormal. But what is the function of anchoring the nucleus to the posterior cortex. The team speculated it might have a relationship with asymmetric localization of another factor, POP-1, which is linked with distinct transcriptional activity and cell fate determination. By measuring the proximity of the nucleus to the cell cortex in mutants lacking a protein required for centrosomes attachment to the cortex, they found that POP-1 asymmetry could be maintained even in the absence of nuclear anchoring. But on looking more closely, using mutants in which asymmetric cell fate determination and POP-1 localization were only partially defective, he was able to show a strong correlation between the proximity of the nucleus to the cortex with POP-1 aysmmetry, suggesting that, while nuclear cortical attachment itself is not essential, it may support normal asymmetry by holding the nucleus close to the cell cortex. “We know that, in many organisms, the nucleus is often found off-center, but the underlying mechanisms and significance are for the most part still unknown,” says Sugioka. “By showing how an extracellular signal can drive nuclear localization, and how the positioning of the nucleus near the cortex makes it more accessible to signals, we have added what I think will be an important piece to this puzzle.”
|
||||
|
||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |