| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
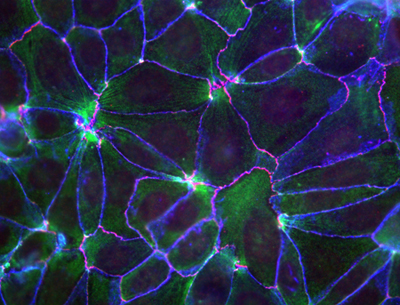
May 25, 2010 – Near their apical surfaces, epithelial cells are bound to their neighbors through molecular interactions that collectively give rise to an active space known as the adherens junction. In addition to holding cells of the same type in close proximity, these junctions are believed to act as sensors for mechanical forces pushing and tugging at the cellular membrane, allowing it to respond and maintain its shape and integrity through rearrangements of the cytoskeleton. It is suspected that cytoplasmic elements that attach to cadherins, the primary cell-cell adhesion molecules, are responsible for this regulatory activity, but recent research has cast doubt on the most popular contender, a protein called α-catenin, as the elusive molecule behind this mechanism.
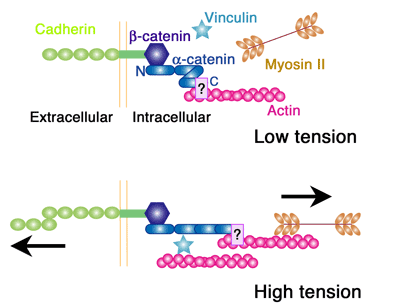
Now, however, a study by Shigenobu Yonemura, head of the Electron Microscope Laboratory, and colleagues has reinstated α-catenin as the leading candidate for the link between the adhesion machinery and the cytoskeleton. This latest work reveals that the protein recruits a second factor, vinculin, known to interact with the cytoskeletal molecule actin in a manner determined by mechanical forces. The key molecular complex in the adherens junction is known as the cadherin-catenin complex, which forms when a group of catenin molecules (β,α, and p120) attaches to the cytoplasmic tail of any one of the many classic cadherin adhesion molecules. α-catenin is also known to bind with actin filaments and multiple actin-binding elements (including vinculin), which seemingly made it a natural candidate as the linker between cell adhesion and the cytoskeleton. But recent evidence suggesting that α-catenin does not simultaneously bind both the cadherin complex and actin filaments in vivo put its role in doubt. Intrigues by this problem, Yonemura and colleagues looked at a number of epithelial cell types in vitro for clues into the mechanism. Observing that vinculin (but not α-catenin) accumulates at adherens junctions only in the presence of the force-generating protein myosin II, the team conjectured that α-catenin’s binding to vinculin might be dependent on the activity of myosin II. Dissection of the protein’s molecular structure revealed three active regions: the amino terminus binds β-catenin, the carboxy end binds various actin-binding factors, while vinculin binding occurs in the central region. Experimenting with various combinations of the presence and absence of α-catenin and force generation, Yonemura found that, contrary to the original speculation, vinculin molecules do not bind α-catenin in a force-dependent manner, but instead that a central region of the protein inhibits vinculin binding and that the carboxy terminus is required for the force-dependent alleviation of this inhibition.
Seeking to pinpoint its functional regions, the team generated a series of α-catenin mutants lacking various regions, and identified the vinculin-binding region (residues 325 – 360) and the region responsible for releasing the inhibition of vinculin binding (residues 697 – 906). From this structural understanding, Yonemura developed a model for force-dependent binding between α-catenin and vinculin, in which the application of mechanical force exposes the region of the α-catenin protein that releases the inhibition of vinculin, allowing its recruitment. Further examination of α-catenin dynamics in the absence and presence of cytoskeleton-generated forces revealed that its turnover was lower in presence of myosin II activity (which generates force), in line with predictions from the functional model. And, importantly, the force-dependent mechanism was shown to be in effect in membrane-bound α-catenin as well, indicting that even when it is functioning as part of the cadherin-catenin complex, α-catenin is still capable of responding to forces.
|
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |