| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
September 1, 2010 – The diversity of cell types in the animal body is the result processes such asasymmetric cell division, in which a mother cell divides mitotically to give rise to two daughters of different character. The axis of asymmetric cell division is critical for determining the future positions of the differentiated progeny in developing embryos. These processes have been intensively studied in models such as the developing nervous system in the fruit fly and in the earliest stages of roundworm embryogenesis. Despite the importance of these phenomena and the scientific attention paid to them, a number of fundamental questions remain. Particularly controversial among these is the problem of whether various events that occur during asymmetric division are regulated by extrinsic or intrinsic mechanisms.
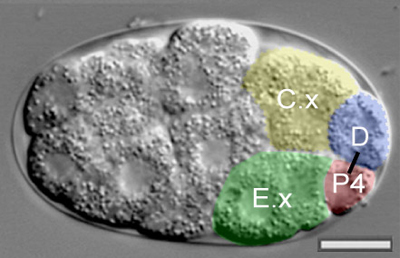
Yukinobu Arata and others in the Laboratory for Cell Fate Decision (Hitoshi Sawa, Team Leader), working in collaboration with scientists from the University of North Carolina at Chapel Hill, have now addressed this question in fine detail with respect to some of the earliest cell divisions in the C. elegans embryo. In a report published in the journal Development, they show that while the asymmetric distribution of the protein PAR-2 occurs in a cell-autonomous manner, its localization to the point of cell-cell contact relies on external signals mediated by the transmembrane protein MES-1 and tyrosine kinase SRC-1. Arata and colleagues focused on a set of germline precursor cells (P0 through P4) that arise in successive rounds of asymmetric cell division in the very early C. elegans embryo, and their interactions with neighboring cells. One important characteristic of the P cell lineage is that the axis of the P3 and P4 polarity is in the reversed orientation to that of the P0 and P1 cells. This polarity reversal allows the P2 cell to give rise to a P3 daughter in contact with the endodermal precursor E, and for P3 to generate a P4 cell that is also in contact with an endodermal precursor, E.x. PAR polarity proteins are known to localize to the points of contact between the germline and endodermal precursor cells, suggesting extracellular signals are at work. However, some previous studies had suggested that the regulation of the polarity reversal that takes place between the P2 and P3 divisions is cell-intrinsic. The team sought to gain a better understanding of this process by isolating individual P-lineage cells from developing embryos in vitro using glass micropipettes. They were surprised to find that the P4 cells deriving from isolates were frequently in inverted position, suggesting that the P3 division axis might rely on extracellular cues. To test this possibility, Arata altered the contact site by isolating cells and attaching germline precurors with either endodermal precursor cells again and watched for effects on the division axis. The results clearly showed that endodermal precursor cells exercise a clear influence on P cell disposition, indicating the axis of division is determined by extracellular signals. The daughters of P2 and 3 cells attached to control cells, however, showed highly variable division axes, highlighting the necessity of external inputs in axis orientation. They used a similar strategy to look next at protein distribution and centrosome positioning. In P cells exposed to both endodermal precursor and control cells, asymmetric distribution of PAR proteins occurred, but interestingly the localization of these proteins to the point of cell-cell contact failed to take place in P cells attached to controls. Simultaneously, the centrosome in P cells in contact with endodermal lineage cells consistently located adjacent to the site of contact between cells, but when control cells were used, the centrosome location was variable, suggesting that external cues from the endodermal cells determine the orientation of asymmetric cell division by regulating both PAR protein distribution and centrosome orientation. The molecules responsible remained unknown but, based on previous reports of the role of two factors – MES-1 and SRC-1 – in establishing differential daughter cell sizes, the team investigated the possibility that they might be involved in axis determination as well. Embryos with losses of function in mes-1 and src-1 they found that the PAR-2 protein was asymmetrically distributed as usual, but that it often failed to accumulate at the cell-cell contact site indicating that these genes shows clear defects in axis orientation. And lastly, to determine whether MES-1 and SRC-1 were required in the P lineage cell or its endodermal neighbor, they tested various combinations of chimeric pairs of P and endodermal cells using cells isolated from mutants and wild type embryos, and found that while the transmembrane protein MES-1 plays an essential part on both sides of the cell-cell junction, the tyrosine kinase SRC-1 is needed only in the signal-receiving P cell. This is the first experimental demonstration in which cell positions were manipulated to directly test the role of cell signaling on directional control of PAR protein localization during asymmetric cell division, work that may shed new light on the directional control of cell polarization in many other developmental processes. “I observed cell divisions of cells isolated by micropipette from developing embryos in vitro, and I have to say that the I never got tired of watching them – they’re quite beautiful,” says Arata. “This study began when I noticed that the progeny of P2 and P3 were sometimes in positions opposite to their normal ones. I wanted to find out why.”
|
|||||||
|
|||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |