| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
September 18, 2010 – The cerebellum is one of the largest regions of the mammalian brain, and is responsible for controlling motor and other bodily functions. These functions are enabled by the activity of a distinct group of large and highly arborized cells known as Purkinje neurons, which integrate multiple inputs from other parts of the brain through their many dendrites and send inhibitory signals to cerebellar nuclei involved in motor coordination. The loss of Purkinje cell function is associated with a severe form of ataxia (loss of motor function), making this cell type one of tremdendous potential both for the study of disease and the development of future clinical applications. Attempts to generate Purkinje neurons from stem cell sources at high efficiencies in vitro, however, have proven difficult.
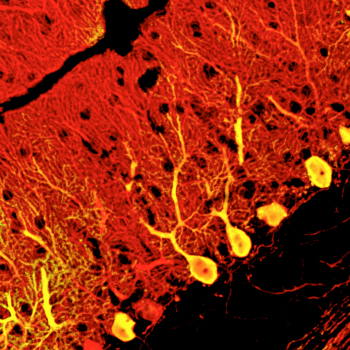
Keiko Mugurmua and colleagues in the Laboratory for Organogenesis and Neurogenesis (Yoshiki Sasai, Group Director), working in collaboration with scientists from the KAN Institute and Kyoto University, have now achieved a breakthough in Purkinje cell induction, selectively deriving these neurons from mouse embryonic stem (ES) cells. In a report published in Nature Neuroscience, the group show how by recapitulating aspects of the cerebellar developmental environment, they were able to generate functional Purkinje cells in quantity. The work began using a technique for neuronal induction using serum-free culture of embryoid body-like aggregates (SFEBq) previously developed by the group and applied to the differentiation of numerous neural cell types. In order to adapt this protocol to Purkinje induction, they looked to how these neurons are induced to develop in the embryo itself. Purkinje cell precursors are induced by signals from a region in the posterior midbrain known as the isthmic organizer. By simulating this structure in cultured cells, Muguruma sought to give rise secondarily to Purkinje neurons. Using a trial and error approach, she tested various transcription factors at different timepoints and in different orders, before finally arriving at a method for inducing cerebellar tissue. This involves SFEBq-culturing mouse ES cells for 1-2 days before adding the caudalizing factors insulin and FGF2, yielding an isthmic organizer-like tissue after 4 days. This region secretes inductive factors, which over time lead to the differentiation of cerebellar precursors at a very high efficiency of around 80%. Subsequent addition of an inhibitor of the ventralizing factor hedgehog to these precursors at day 13 gives rise to Purkinje neurons at a rate of around 30% of all cells by day 19. Subjecting this population to cell sorting by flow cytometry allows for even greater purities, of up to about 90%. The Purkinje neurons derived through this approach were similar in morphology and surface marker expression, but would they function in the same way as their embryonic equivalents? Muguruma introduced ES-derived Purkinje cells into the cerebella of day 16 mouse embryos to find out. Allowing the transplanted embryos to develop to term, she found that their cerebellar tissues contained many such cells, testifying to the power of the new technqiue. The transplanted Purkinje neurons not only integrated into the tissue, but also formed functional nerual circuits, and exhibited normal electrophysiology and neuronal inputs. “It is still early days,” says Group Director Sasai, “but if we are able to derive populations of human cerebellar neurons in a similar fashion, these might be of great benefit to the study and treatment of diseases like spinocerebellar ataxia in the future.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |