| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
November 15, 2010 – Asymmetric cell division, in which a single mother cell divides to give rise daughters with different properties such as gene expression and morphology, is a fundamental mechanism by which cell diversity is generated. This phenomenon has been extensively studied in the nervous system of the fruit fly Drosophila melanogaster, in which specific protein factors are allocated to the basal side of dividing neuronal progenitors, steering the cell that inherits these basal determinants to a ganglion mother cell (GMC) fate, while its mitotic sibling becomes a larger neuroblast. Past studies have shown that daughter cell characteristics such as cell size and protein localization are under the control of independent molecular mechanisms. But the donwstream consequences of the loss of asymmetry in these features remain something of an open question.
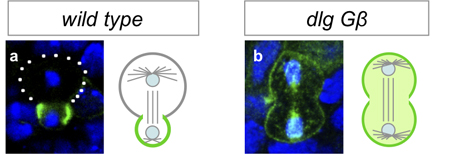
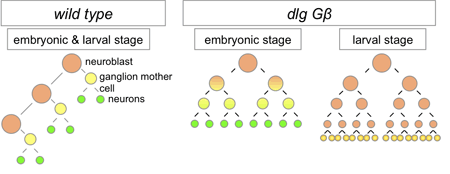
Working with mutants for a pair of genes linked to the maintenance of asymmetric mitosis, Atsushi Kitajima and others in the Laboratory for Cell Asymmetry (Fumio Matsuzaki, Group Director), in colaboration with the National Institute of Genetics, observed how the loss of these functions leads to abnormal progeny. The study, published in Developmental Biology, reveals in these mutants that, in embryos, the daughter cells that would normally form neuroblasts instead differentiate into neurons. Kitajima began by generating flies carrying mutations for Gß13 (which is associated with maintaining differential sizes in the daughters of neural progenitors) and dlg (an oncogene linked with controlling the asymmetric intracellular localization of fate determinants). As expected, neuroblast progeny in these cells showed abnormal symmetry in cell size and equal distribution of determinant proteins. The group next monitored the fate of cells bron from the mutant neuroblasts in embryos, and determined that while the newborn daughters bore the hallmarks of neuroblasts and GMCs, the stem cell nature of the progeny neuroblasts was not maintained, as most ultimately differentiated into neurons. Asymmetric cell division is seen not only at embryonic stages, but in larval flies as well. Using a conditional knockout to interfere with Gß13 and dlg function in specific tissues and observe the lineage effects. Interestingly, Kitajima et al. found that unlike in the embryo, the double-mutant cells failed to differentiate, and proliferated abnormally instead in a maner similar to that seen in mutants of the basal determinant Prospero (itself an oncogene). The authors speculate that the larger size of the mutant progeny may prevent a sufficient amount of Prospero from being allocated to a single daughter (the presumptive GMC), leading to misregulation of cell proliferation in larval neural tissue, a surmise that was borne out by the finding that overexpression of Prospero in the double-mutants rescued the phenotype.
“It is intriguing how symmetrically dividing stem cells behave so differently in embryonic and larval contexts,” comments Matsuzaki. “We see how the same combination of mutations can lead to terminal differentiation in once milieu, but tumor-like over-proliferation in another. In the future, we hope to determine whether this holds true in other species as well.” |
|||||||
|
|||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |